- EN - English
- CN - 中文
Generation, Propagation, and Titering of Dicistrovirus From an Infectious Clone
感染克隆生成、扩增和滴度测定蝗虫麻痹病毒
发布: 2025年02月20日第15卷第4期 DOI: 10.21769/BioProtoc.5216 浏览次数: 2185
评审: Keisuke TabataChhuttan L MeenaAnonymous reviewer(s)

相关实验方案
诱导型HIV-1库削减检测(HIVRRA):用于评估外周血单个核细胞中HIV-1潜伏库清除策略毒性与效力的快速敏感方法
Jade Jansen [...] Neeltje A. Kootstra
2025年07月20日 2457 阅读
Abstract
Cricket paralysis virus (CrPV), a member of the family Dicistroviridae, is a single-stranded positive-sense RNA virus that primarily infects arthropods. Some members of the dicistrovirus family, including the honey bee viruses Israeli acute paralysis virus and Acute bee paralysis virus and the shrimp-infecting Taura syndrome virus, pose significant threats to agricultural ecosystems and economies worldwide. Dicistrovirus infection in Drosophila is used as a model system to study fundamental insect–virus–host interactions. The availability of a CrPV infectious clone allows controlled manipulation of the viral genome at a molecular level. Effective viral propagation and titration techniques are crucial for understanding the pathogenesis and epidemiology of dicistrovirus infections. Traditional methods for assessing viral titers, such as plaque assays, are unsuitable for CrPV, since Drosophila tissue culture cells like Schneider 2 cells cannot readily form adherent plaques. Here, we present a streamlined protocol for generating a recombinant virus from a CrPV infectious clone, propagating the virus in S2 cells and titering the virus by an immunofluorescence-based focus-forming assay (FFA). This protocol offers a rapid and reliable approach for generating recombinant viruses, viral amplification, and determining CrPV titers, enabling efficient investigation into viral biology and facilitating the development of antiviral strategies.
Key features
• Generate recombinant virus from infectious clones.
• Sequential amplification protocol for scalable virus production.
• Repeated freeze-thawing for virus harvesting.
• Immunostaining focus-forming assay (FFA) for CrPV titration.
• Focus-forming units (FFU) quantified using a high-throughput microscopic screening platform.
Keywords: Cricket paralysis virus (蝗虫麻痹病毒)Graphical overview
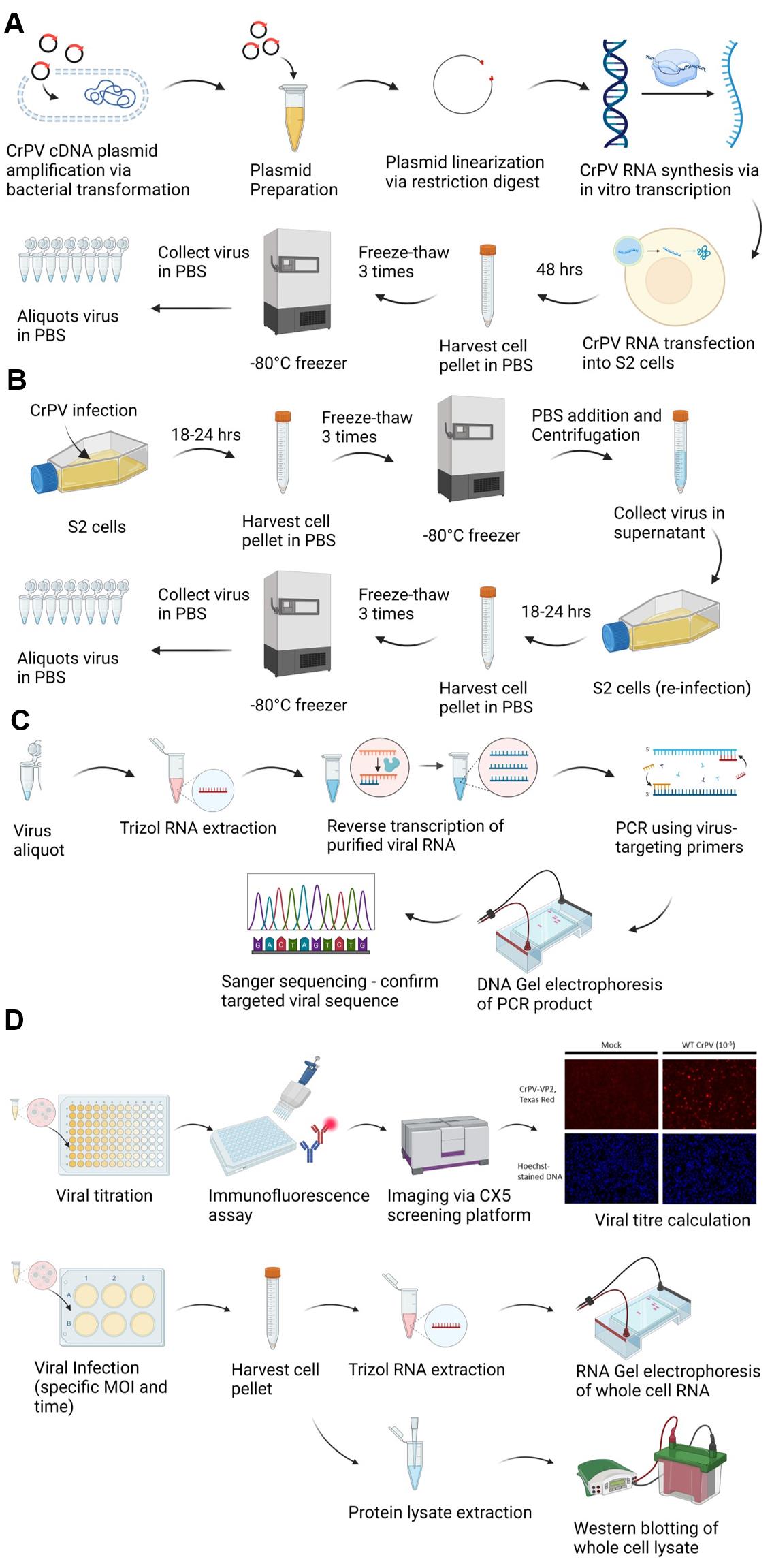
Graphical overview of cricket paralysis virus (CrPV) infectious clone generation, propagation, sequence confirmation, titering, and infection. A. CrPV infectious clone preparation and propagation. CrPV cDNA plasmid is amplified via bacterial transformation, linearized by restriction enzyme digestion, and used in an in vitro transcription reaction to produce CrPV genomic RNA. S2 cells are transfected with the CrPV RNA and then incubated for 48 h to allow viral protein synthesis and viral replication. Infected cells are harvested and subjected to three freeze-thaw cycles to release intracellular virus, and the viral stock is aliquoted and stored at -80 °C. B. Propagation of CrPV in S2 cells via sequential amplification. S2 cells are infected with CrPV and incubated for 18–24 h. After incubation, cells are harvested and subjected to three freeze-thaw cycles to release the virus. The harvested virus is used to reinfect fresh S2 cells in a sequential amplification step to increase viral yield. The virus is collected from the supernatant and aliquoted for storage at -80 °C for further experiments. C. CrPV genome validation. Viral RNA is purified from aliquots of CrPV, reverse transcribed, and then PCR amplified using CrPV-specific primers. The PCR product is analyzed by DNA gel electrophoresis and verified by Sanger sequencing. D. Titering CrPV. Serial dilutions of the virus (10-fold dilution) are used to infect S2 cells for 6 h and then immunostained with anti-CrPV capsid protein (VP2) antibody followed by secondary staining using Texas red-conjugated antibodies. The infected cells are visualized and quantified using a high-throughput microscopic screening platform. The viral titer is calculated based on the number of VP2-positive cells. D. RNA gel electrophoresis and western blotting for infection validation. S2 cells are infected with CrPV at specific multiplicity of infection (MOI) values and harvested after defined time points. RNA is extracted from the infected cells and analyzed via RNA gel electrophoresis and western blotting targeting CrPV-1A and CrPV-VP2 to assess the progression of the infection.
Background
Cricket paralysis virus (CrPV), a small RNA virus from the Dicistroviridae family, primarily infects arthropods, including economically significant pests and the model organism Drosophila melanogaster [1,2]. The genome organization of CrPV includes two internal ribosome entry sites (IRESs) that direct the translation of non-structural and structural protein-encoding open reading frames (ORF1 and ORF2), as shown in the schematic representation of the CrPV genome (Figure 1) [2]. The availability of an infectious CrPV clone has proven invaluable for introducing precise mutations into viral RNA and proteins, allowing researchers to investigate the specific roles of individual viral components during infection [3–6]. As CrPV serves as a model system for studying fundamental virus–host interactions, the development of efficient methods for propagating and quantifying the virus is critical. Traditional viral titration methods, such as plaque assays, involve counting plaques formed by the lysis of virus-infected cells. However, Drosophila S2 cells are semi-adherent and cannot form the dense monolayers required for plaque formation by CrPV. As a result, CrPV-infected cells fail to produce clear, countable plaques, making them unsuitable for standard plaque assays. This limitation highlights the need for alternative methods to accurately and efficiently quantify CrPV.
Our protocol for CrPV propagation and titration addresses these challenges. A sequential amplification approach is used, wherein an initial infection of Drosophila S2 cells (30 million) is performed. Subsequently, the intracellular virus is harvested via freeze-thaw cycles and used to infect a larger batch of cells (100 million) to amplify the virus. This step helps produce higher viral yields by harvesting the cells before lysis. Finally, infected cells are then subject to freeze-thaw to disrupt cellular membranes to release the accumulated intracellular viral particles. This method helps with the continuous amplification of the virus.
For viral titration, we use an immunostaining-based focus-forming assay (FFA), a limiting dilution assay that quantifies the number of infectious particles in a given volume. Viral stocks are serially diluted and used to infect S2 cells in a 96-well plate that has been pre-treated with Concanavalin A to enhance cell adherence [7]. After incubation, cells are fixed with paraformaldehyde, permeabilized with methanol, and stained with antibodies against the CrPV capsid protein, VP2. The fluorescence signal shows the presence of VP2 expression within cells, serving as an indicator of the infection status. Fluorescence microscopy, such as using a high-throughput imaging system (e.g., CellInsightTM CX5 High Content Screening), is used to visualize and quantify the infected cell foci, expressed as focus-forming units (FFU) per milliliter. This immunostaining-based titration method can be effectively used to determine viral titer when plaque assay is not an option. By providing a reliable method for virus propagation and titration, our protocol advances the study of insect viruses, contributing to the broader understanding of viral biology and the development of novel antiviral strategies.
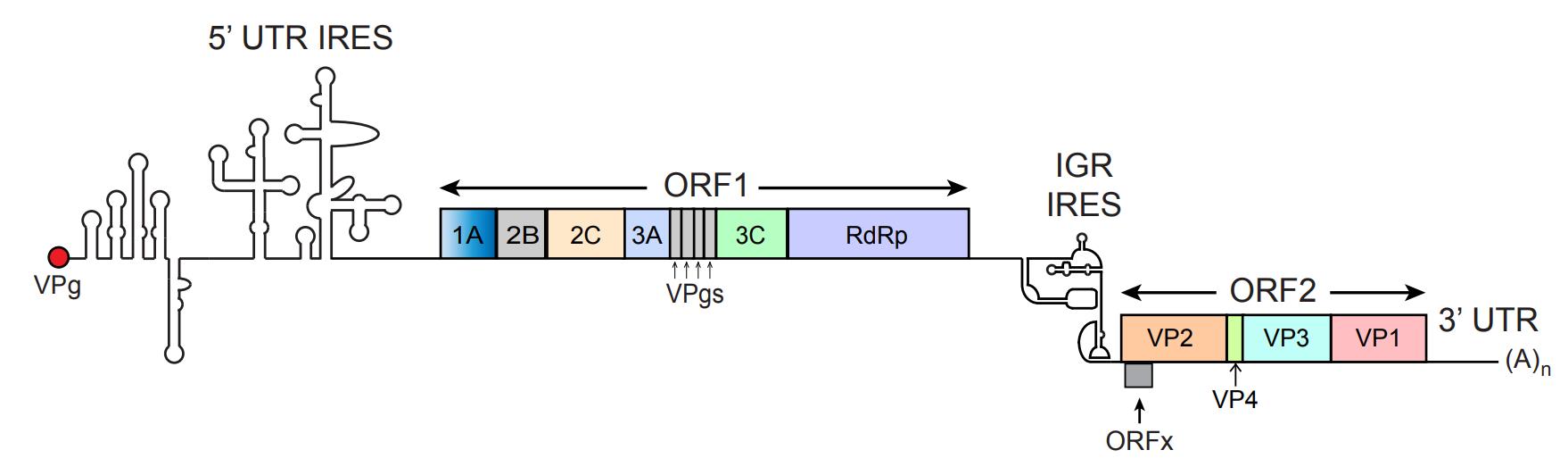
Figure 1. RNA genome of cricket paralysis virus (CrPV). CrPV is a positive-sense, single-stranded RNA. It contains two internal ribosome entry sites (IRESs) that facilitate the translation of two distinct open reading frames (ORF1 and ORF2). The 5' UTR IRES initiates translation of ORF1, which encodes non-structural proteins involved in viral replication, including 1A, 2B, 2C, 3A, 3B, 3C, and the RNA-dependent RNA polymerase (RdRp or 3D), as well as four viral protein genomes (VPgs). The intergenic region (IGR) IRES initiates the translation of ORF2, which encodes structural proteins essential for forming the viral capsid: VP2, VP3, VP1, and VP4. The CrPV genome is flanked by a 5' UTR with an IRES structure and a 3' UTR that ends with a poly(A) tail, which are both crucial for viral replication and translation efficiency. This figure is adapted from Warsaba et al. [2], Dicistrovirus-Host Molecular Interactions, Current Issues in Molecular Biology.
Materials and reagents
Biological materials
1. Cricket paralysis virus (CrPV) infectious clone, generated based on the methodology described in the Journal of Virology article titled "The 5' Untranslated Region of a Novel Infectious Molecular Clone of the Dicistrovirus Cricket Paralysis Virus Modulates Infection" by Kerr et al. [3]
2. 5-alpha competent E. coli (New England Biolabs, catalog number: C2987)
3. Drosophila Schneider 2 (S2) cells cultured in Schneider's Drosophila medium (Thermo Fisher Scientific, catalog numbers: R69007)
Reagents
1. 1 kb plus DNA ladder (New England Biolabs, catalog number: N3232)
2. 100 bp DNA ladder (New England Biolabs, catalog number: N3231)
3. Acetic acid (Sigma-Aldrich, catalog number: 45754)
4. Agar (Sigma-Aldrich, catalog number: 05040)
5. Ammonium persulfate (APS) (Sigma-Aldrich, catalog number: A3678)
6. Ampicillin sodium salt (Sigma-Aldrich, catalog number: A8351)
7. α-CrPV-1A antibody (custom protein antibody, Genscript) [5]
8. α-CrPV-VP2 antibody (custom peptide antibody, Genscript – C-ATFQDKQENSHIENE-NH2 and C-KLWIHKTYLKRPAR-NH2) [8]
9. Bleach/sodium hypochlorite solution (Sigma-Aldrich, catalog number: 1.05614)
10. Blue protein loading dye (New England Biolabs, catalog number: B7703)
11. Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7906)
12. Bradford protein assay dye reagent concentrate (Bio-Rad, catalog number: 5000006)
13. Chemiluminescence kit (Thermo Fisher Scientific, catalog number: 89880)
14. Chloroform (Thermo Fisher Scientific, catalog number: 043685.M6)
15. Concanavalin A (Con A) (Sigma-Aldrich, catalog number: 11028-71-0)
16. Diethyl pyrocarbonate (DEPC) (Sigma-Aldrich, catalog number: D5758)
17. Donkey anti-rabbit IgG-horseradish peroxidase (Amersham, catalog number: NA934)
18. DNase I (New England Biolabs, catalog number: M0303)
19. Ecl136II restriction enzyme (Thermo Fisher Scientific, catalog number: ER0251)
20. EDTA (ethylenediaminetetraacetic acid, disodium salt) (Sigma-Aldrich, catalog number: 798681)
21. Ethanol (Sigma-Aldrich, catalog number: 1.00974)
22. Fetal bovine serum (FBS) (Gibco, catalog number: 16-000-069)
23. Gel loading dye purple (6×) (New England Biolabs, catalog number: B7024)
24. Glacial acetic acid (Sigma-Aldrich, catalog number: 1005706)
25. Glycerol (Sigma-Aldrich, catalog number: G7893)
26. Goat anti-mouse IgG-horseradish peroxidase (Santa Cruz Biotechnology, catalog number: sc-2005)
27. Guanidine hydrochloride (Sigma-Aldrich, catalog number: G3272)
28. Halt protease and phosphatase inhibitor cocktail (100×) (Thermo Fisher Scientific, catalog number: 78444)
29. Hoechst (Invitrogen, catalog number: H21486)
30. IGEPAL CA-630 (Nonidet P-40, NP-40 substitute) (Sigma-Aldrich, catalog number: 56741)
31. Isopropanol (Thermo Fisher Scientific, catalog number: 040983.M1)
32. Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific, catalog number: 11668027)
33. LunaScript RT supermix kit (New England Biolabs, catalog number: E3010)
34. Methanol (Fisher Scientific, catalog number: 67-56-1)
35. Milk powder (Sigma-Aldrich, catalog number: 1443825)
36. Molecular-grade agarose (FroggaBio, catalog number: A87-500G)
37. Monarch PCR & DNA Cleanup kit (5 μg) (New England Biolabs, catalog number: T1030)
38. MOPS [3-(N-morpholino)propanesulfonic acid] (Sigma-Aldrich, catalog number: 475898)
39. Gibco Opti-MEM reduced serum medium (Fisher Scientific, catalog number: 31985070)
40. Paraformaldehyde (Fisher Scientific, catalog number: AC416785000)
41. Penicillin G potassium salt (Sigma-Aldrich, catalog number: 113-98-4)
42. Phusion High-Fidelity PCR kit (New England Biolabs, catalog number: E0553)
43. Potassium chloride (Sigma-Aldrich, catalog number: 529552)
44. Potassium phosphate monobasic (Sigma-Aldrich, catalog number: 529568)
45. Qubit RNA High-Sensitivity (HS) Assay kit (Invitrogen, catalog number: Q32855)
46. Riboruler RNA ladder (Thermo Fisher Scientific, catalog number: SM1821)
47. RiboLock RNase inhibitor (Thermo Fisher Scientific, catalog number: EO0382)
48. Ribonucleotide solution mix (New England Biolabs, catalog number: N0466)
49. RNA Clean-Up kit (New England Biolabs, catalog number: T2040)
50. SafeView DNA stains (Applied Biological Materials, catalog number: G108)
51. SalI restriction enzyme (New England Biolabs, catalog number: R0138)
52. Schneider's Drosophila medium (Gibco, catalog number: 21720024)
53. Sodium chloride (Sigma-Aldrich, catalog number: S9888)
54. Sodium deoxycholate (Sigma-Aldrich, catalog number: 264103)
55. Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: 11667289001)
56. Sodium phosphate dibasic (Sigma-Aldrich, catalog number: 567547)
57. Streptomycin sulfate salt (Sigma-Aldrich, catalog number: 3810-74-0)
58. T7 RNA polymerase (New England Biolabs, catalog number: E2040S)
59. TEMED (Sigma-Aldrich, catalog number: T7024)
60. Texas red goat anti-rabbit IgG (H+L) secondary antibody (Invitrogen, catalog number: T-2767)
61. Tris base (Sigma-Aldrich, catalog number: 252859)
62. Tris hydrochloride (Sigma-Aldrich, catalog number: 10812846001)
63. Triton X-100 (Sigma-Aldrich, catalog number: X100RS)
64. Trizol (Thermo Fisher Scientific, catalog number: 15596026)
65. Tryptone (Sigma-Aldrich, catalog number: T2559)
66. Tween 20 (Sigma-Aldrich, catalog number: P1379)
67. Yeast extract (Sigma-Aldrich, catalog number: Y1625)
Solutions
1. Ampicillin stock solution (see Recipes)
2. Complete Schneider's Drosophila medium (see Recipes)
3. Con A solution (see Recipes)
4. DEPC-treated water (see Recipes)
5. LB-agar plate (see Recipes)
6. Liquid LB (see Recipes)
7. Penicillin and streptomycin solution (see Recipes)
8. LB-gar solution (see Recipes)
9. LB solution (see Recipes)
10. PB buffer (DNA purification) (see Recipes)
11. PE buffer (wash buffer) (see Recipes)
12. Phosphate buffered saline (PBS) solution (see Recipes)
13. 1× Tris-Acetate-EDTA (TAE) buffer(see Recipes)
14. Radio-immunoprecipitation assay (RIPA) buffer (see Recipes)
15. 10× MOPS (3-(N-morpholino)propanesulfonic acid) buffer (see Recipes)
16. 0.1% Tris-buffered saline with Tween 20 (TBST) buffer (see Recipes)
17. Resolving and stacking solutions for western blotting: made right before gel casting
18. Texas red secondary antibody in 5% BSA/PBS: it is light-sensitive; wrap with aluminum foil to prevent photobleaching
Recipes
1. Ampicillin stock solution
Dissolve ampicillin salts in DEPC-treated water to 100 mg/mL. This solution can be aliquoted to 1 mL per microfuge tube and stored at -20 °C.
2. Complete Schneider's Drosophila medium
Schneider's Drosophila medium includes 10% FBS and 1% penicillin and streptomycin solution. After mixing, filter the media using a bottle-top vacuum filter and store in an autoclaved glass bottle at 4 °C. It can be stored indefinitely if the bottle is unopened.
3. Con A solution
Con A in PBS (0.5 mg/mL), 3% paraformaldehyde, and 5% BSA in PBS. Filter by a syringe filter and store on ice.
4. DEPC-treated water
Treat Milli-Q water with 0.2% (v/v) DEPC and autoclave for 30 min in a glass bottle. Cool down the bottled water to room temperature before use. It can be stored indefinitely if the bottle is unopened.
5. LB-agar plate
Place a magnetic stir bar into the LB-agar solution and autoclave it in a glass bottle for 30 min at 121 °C. After autoclaving, stir the solution on a magnetic stir plate while allowing it to cool to approximately 50 °C. Once cooled, add ampicillin to a final concentration of 100 μg/mL and mix gently to ensure even distribution. Pour the LB-agar into Petri dishes, adding just enough to cover the bottom of each dish. Close the lids and allow the plates to cool and solidify overnight at room temperature.
6. Liquid LB
Autoclave LB solution for 30 min in a glass bottle and store at room temperature. Cool down the bottled water to room temperature before use.
7. Penicillin and streptomycin solution
Dissolve penicillin and streptomycin salts in DEPC-treated water to 6 mg/mL and 10 mg/mL, respectively. This solution can be aliquoted to 10 mL per 15 mL Falcon tube and stored at -20 °C.
9. LB-agar solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tryptone | 1% (w/v) | 10 g |
| Yeast extract | 0.5% (w/v) | 5 g |
| Sodium chloride | 1% (w/v) | 10 g |
| Agar | 2% (w/v) | 20 g |
| Deionized water (dH2O) | n/a | To 1 L |
| Total | n/a | 1 L |
10. LB solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tryptone | 1% (w/v) | 10 g |
| Yeast extract | 0.5% (w/v) | 5 g |
| Sodium chloride | 1% (w/v) | 10 g |
| Deionized water (dH2O) | n/a | To 1 L |
| Total | n/a | 1 L |
11. PB buffer (DNA purification)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Guanidine hydrochloride (GuHCl) | 5 M | 476.6 g |
| Isopropanol | 30% (v/v) | 300 mL |
| Tris-HCl (pH 6.6) | 20 mM | 2.42 g |
| Deionized water (dH2O) | n/a | To 1 L |
| Total | n/a | 1 L |
12. PE buffer (wash buffer)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris-HCl | 10 mM | 1.21 g |
| Ethanol | 80% (v/v) | 800 mL |
| Deionized water (dH2O) | n/a | To 1 L |
| Total | n/a | 1 L |
13. Phosphate buffered saline (PBS) solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sodium chloride | 137 mM | 8 g |
| Potassium chloride | 2.7 mM | 0.2 g |
| Sodium phosphate dibasic | 10 mM | 1.44 g |
Potassium phosphate monobasic Deionized water (dH2O) | 1.8 mM n/a | 0.24 g To 1 L |
| Total | n/a | 1 L |
Note: Autoclave for 30 min in a 1 L glass bottle and store at room temperature.
14. 1× Tris-Acetate-EDTA (TAE) buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris base | 40 mM | 4.84 g |
| Glacial acetic acid | 20 mM | 1.14 mL |
| EDTA | 1 Mm | 0.372 g |
| Deionized water (dH2O) | n/a | To 1 L |
| Total | n/a | 1 L |
Note: Store at room temperature.
15. RIPA buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sodium chloride | 150 mM | 0.439 g |
| IGEPAL CA-630 | 1% | 0.5 mL |
| Sodium deoxycholate | 0.5% | 0.25 g |
| SDS | 0.1% | 0.05 g |
| Glycerol | 10% | 5 mL |
| Tris-HCl (pH 8.0) Tris(hydroxymethyl)aminomethane | 50 mM | 0.3 g (adjust pH to 8) |
| Deionized water (dH2O) | n/a | To 50 mL |
| Total | n/a | 50 mL |
Note: Store at 4 °C and preferably protected from light.
16. 10× MOPS buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| MOPS | 200 mM | 41.86 g |
| Sodium acetate | 50 mM | 4.1 g |
| EDTA | 10 mM | 3.72 g |
| Deionized water (dH2O) | n/a | To 1 L |
| Total | n/a | 1 L |
17. 0.1% Tris-buffered saline with Tween 20 (TBST) buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris base | 20 mM | 2.42 g |
| Sodium chloride | 150 mM | 8 g |
| Tween 20 | 0.1% | 1 mL |
| Deionized water (dH2O) | n/a | To 1 L |
| Total | n/a | 1 L |
Laboratory supplies
1. 1.5, 15, and 50 mL tube rack
2. 1.5 and 2 mL Eppendorf tubes (Thermo Fisher Scientific, catalog numbers: AM12450 and AM12475)
3. 15 and 50 mL Falcon tubes (FroggaBio, catalog numbers: TB15-500 and TB50-500)
4. 14 mL round-bottom test tubes (Sigma-Aldrich, catalog number: CLS352057)
5. 6-well TC-treated plate (Corning, catalog number: CLS353224)
6. Bottle-top vacuum filter (Corning, catalog number: CLS431097)
7. Centrifuge (Thermo Fisher Scientific, model: accuSpin 1-1R)
8. Disposable polystyrene cuvettes (Bio-Rad, catalog number: 2239955)
9. DNase/RNase-free tips (1,000, 200, 10 μL)
10. Kimwipes (Fisher Scientific, catalog number: 06-666)
11. Micropipettes (200–1,000, 20–200, 2–20, and 0.2–2 μL)
12. Multichannel micropipettes (20–200 μL)
13. PCR strip tubes (FroggaBio, catalog number: STF-A120)
14. Petri dishes (Thermo Fisher Scientific, catalog number: FB0875713)
15. Spin Miniprep columns (Qiagen, catalog number: 27115)
16. Sterile 96-well plate, surface treated (VWR, catalog number: 10062-900)
17. Sterile serological pipettes 10 mL (Fisher Scientific, catalog number: 13-678-12)
18. Sterile serological pipettes 25 mL (Fisher Scientific, catalog number: 13-678-14)
19. Syringe filter (Sigma-Aldrich, catalog number: SLMP025SS)
20. T25 and T75 TC flask (Thermo Fisher Scientific, catalog numbers: 156367 and 156499)
Equipment
1. Isotemp incubator (Thermo Fisher Scientific, catalog number: 151030513)
2. Bio-Rad Gel Doc 2000 (Bio-Rad, catalog number: 04678)
3. BioPhotometer spectrophotometer (Eppendorf, catalog number: 6131-FCP22)
4. CellInsight CX5 HCS platform (Thermo Fisher Scientific, catalog number: CX51110)
5. EVOS FLoid imaging system (Thermo Fisher Scientific, catalog number: 4471136)
6. NanoDrop spectrophotometer ND-1000 (Thermo Fisher Scientific, catalog number: 2353-30-0010)
7. Typhoon biomolecular imager (Cytiva, catalog number: 29187191)
Software and datasets
1. BioRender version 4.0 (BioRender.com, 10/2024)
2. HCS Navigator version 6.6.0 (Thermo Fisher Scientific, 12/2015)
3. ImageJ version 1.54g (National Institutes of Health, Bethesda, MD, USA, 2024)
4. Microsoft Excel for Microsoft 365 (Microsoft Corporation, 2024)
5. SnapGene version 6.0 (GSL Biotech, 2024)
Procedure
文章信息
稿件历史记录
提交日期: Nov 1, 2024
接收日期: Jan 8, 2025
在线发布日期: Jan 22, 2025
出版日期: Feb 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Shen, J., Sadasivan, J. and Jan, E. (2025). Generation, Propagation, and Titering of Dicistrovirus From an Infectious Clone. Bio-protocol 15(4): e5216. DOI: 10.21769/BioProtoc.5216.
分类
微生物学 > 微生物-宿主相互作用 > 病毒
生物化学 > 病毒 > 分离和纯化
微生物学 > 微生物细胞生物学 > 病毒繁殖
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link