- EN - English
- CN - 中文
Transgene-free Genome Editing in Grapevine
葡萄树的无转基因基因组编辑
发布: 2025年02月20日第15卷第4期 DOI: 10.21769/BioProtoc.5190 浏览次数: 2496
评审: Clizia VillanoIgnacio LescanoAnonymous reviewer(s)
Abstract
CRISPR/Cas9 genome editing technology has revolutionized plant breeding by offering precise and rapid modifications. Traditional breeding methods are often slow and imprecise, whereas CRISPR/Cas9 allows for targeted genetic improvements. Previously, direct delivery of Cas9-single guide RNA (sgRNA) ribonucleoprotein (RNP) complexes to grapevine (Vitis vinifera) protoplasts has been demonstrated, but successful regeneration of edited protoplasts into whole plants has not been achieved. Here, we describe an efficient protocol for obtaining transgene/DNA-free edited grapevine plants by transfecting protoplasts isolated from embryogenic callus and subsequently regenerating them. The regenerated edited plants were comparable in morphology and growth habit to wild-type controls. This protocol provides a highly efficient method for DNA-free genome editing in grapevine, addressing regulatory concerns and potentially facilitating the genetic improvement of grapevine and other woody crop plants.
Key features
• Protoplasts are one of the most commonly used systems for the application of new breeding technologies, including DNA-free genome editing.
• Protoplasts are a highly accessible platform by CRISPR-Cas9 ribonucleoparticles through chemical or physical transfection.
• CRISPR-Cas9 ribonucleoparticles avoid the use of both Agrobacterium tumefaciens and plasmids; no stable integration of exogenous DNA occurs.
• The genetic background of DNA-free edited plants regenerated from protoplasts remains unchanged and identical to the original plant.
Keywords: CRISPR/Cas9 (CRISPR/Cas9)Graphical overview
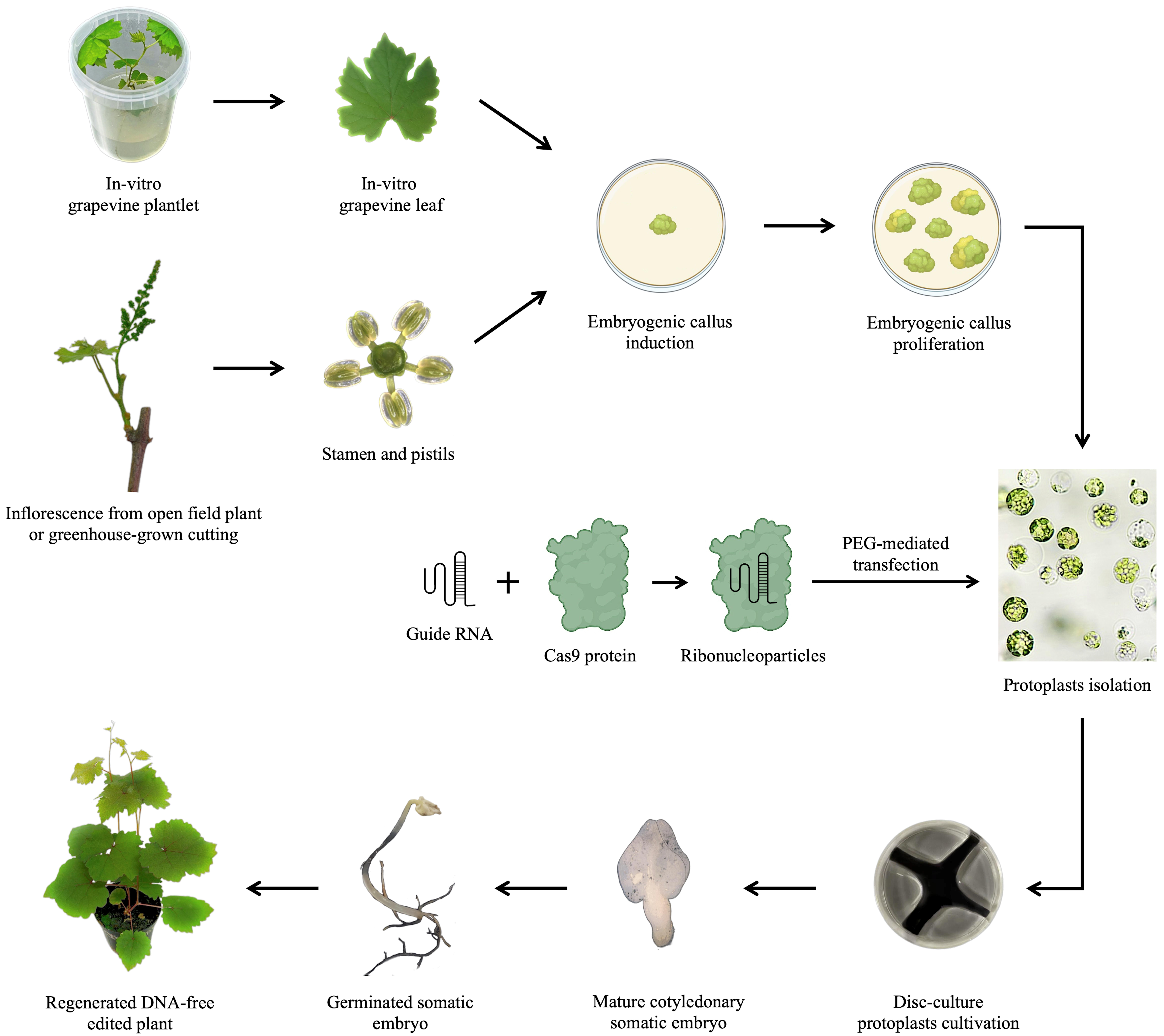
Graphical overview of the workflow of DNA-free genome editing application in embryogenic calli-derived protoplasts and whole plant regeneration. Embryogenic calli are induced from leaves of in vitro plants or from stamens and pistils. After induction and proliferation, embryogenic calli are used for protoplast isolation. Pre-assembled CRISPR-Cas9 ribonucleoproteins (RNPs) are introduced into protoplasts by PEG-mediated transfection. Transfected protoplasts are cultivated using the disc-culture method. The regeneration of whole edited plants from transfected protoplasts occurs through somatic embryo formation.
Background
Grapevine (Vitis vinifera L.) is a significant global fruit crop, valued for its fresh produce and especially for wine. Genetic improvement of grapevine is crucial to address challenges such as climate change, higher demands for quality and quantity, and the need for product differentiation [1,2]. Breeding is a long, time-consuming process, often requiring several years to develop new varieties. Despite the quicker development potential of genetically modified (GM) crops, they encounter opposition from both the public and regulatory agencies due to concerns regarding health and environmental impacts [3]. Nowadays, new breeding technologies, particularly genome editing through the CRISPR/Cas9 system, provide a promising alternative by ensuring precise and targeted genetic modifications without altering the original genetic background. CRISPR/Cas9 uses the Cas9 endonuclease, guided to specific DNA sequences by a single guide RNA (sgRNA), to create site-specific double-stranded breaks. These breaks are typically repaired by non-homologous end joining, resulting in insertions or deletions, or by homology-directed repair if a donor template is available [4]. This method has been used to study and modify various genes in grapevine, enhancing traits such as disease resistance and plant architecture [5–9].
Most CRISPR/Cas9 applications in grapevine have involved stable integration of the editing machinery, thus creating GMOs. To comply with regulations requiring the absence of foreign DNA, two main strategies are used: removing CRISPR/Cas9 components after editing or directly delivering Cas9-sgRNA ribonucleoproteins (RNPs). The latter method avoids foreign DNA integration and has been demonstrated in grapevine protoplasts, but regenerating whole plants from these edited protoplasts has been challenging [10,11]. Recent advancements include both a procedure for regenerating whole plants from embryogenic callus-derived protoplasts in specific grapevine varieties [12] and a method for the regeneration of transgene-free edited grapevine plants from RNP-transfected protoplasts, confirming the feasibility of these approaches for producing normal and healthy transgene-free edited grapevine plants [13]. The advantages of this protocol include avoiding foreign DNA integration, thus addressing regulatory and public concerns associated with GMOs. Additionally, this method reduces the occurrence of genetic chimeras, which are common in organogenesis-based regeneration methods [14,15]. The approach is particularly suitable for varieties recalcitrant to traditional gene transfer methods, offering a versatile tool for grapevine breeding and functional genomics. Potential applications of this protocol extend beyond grapevine to other economically important woody and herbaceous crops. By refining the procedures for protoplast isolation, RNP transfection, and plant regeneration, this method could become a standard for developing transgene-free edited plants. This aligns with regulatory frameworks and facilitates broader acceptance of genome editing technologies in agriculture [16].
Materials and reagents
Biological materials
1. In vitro–grown grapevine plants
2. Inflorescences of open field plants or greenhouse-grown cuttings
Reagents
1. Enzymes
a. Cellulase R10 (Duchefa Biochemie, catalog number: C8001)
b. Macerozyme R10 (Duchefa Biochemie, catalog number: M8002)
c. Pectolyase Y-23 (Duchefa Biochemie, catalog number: P8004)
2. Fluorophores
a. Fluorescein diacetate (FDA) (Sigma-Aldrich, catalog number: 343209)
b. Fluorescent brightener 28 disodium salt (Chemcruz, catalog number: sc-218504)
3. Gelling agents
a. Agar TC (PhytoTech Labs, catalog number: A296)
b. Phytagel (Sigma-Aldrich, catalog number: P8169)
c. Gelrite (Duchefa Biochemie, catalog number: G1101)
4. Growth regulators
a. 6-Benzylaminopurine (6-BAP) (Duchefa Biochemie, catalog number: B0904)
b. 2,4-Dichlorophenoxyacetic acid (2,4-D) (Duchefa Biochemie, catalog number: D0911)
c. α-Naphthalene acetic acid (NAA) (Duchefa Biochemie, catalog number: N0903)
d. β-Naphthoxyacetic acid (NOA) (Duchefa Biochemie, catalog number: N0912)
5. Kit
a. QubitTM RNA BR Assay kit, 500 assays (Invitrogen, Thermo Fisher Scientific, catalog number: Q10211)
b. GeneArt Precision gRNA Synthesis kit (Invitrogen, Thermo Fisher Scientific, catalog number: A29377)
6. Proteins
a. TrueCut TM Cas9 protein v2 (Invitrogen, Thermo Fisher Scientific, catalog number: A36499)
7. Salts and biochemicals
a. CaCl2·2H2O (Duchefa Biochemie, catalog number: C0504)
b. Ca(NO3)2·4H2O (Duchefa Biochemie, catalog number: C0505)
c. KCl (Sigma-Aldrich, catalog number: 31219)
d. KH2PO4 (Duchefa Biochemie, catalog number: P0574)
e. KNO3 (Duchefa Biochemie, catalog number: P0519)
f. K2SO4 (Duchefa Biochemie, catalog number: P0535)
g. MgCl2·6H2O (Duchefa Biochemie, catalog number: M0533)
h. MgSO4·7H2O (Duchefa Biochemie, catalog number: M0513)
i. NaCl (Duchefa Biochemie, catalog number: S0520)
j. NH4NO3 (Duchefa Biochemie, catalog number: A0501)
k. NH4Cl (Duchefa Biochemie, catalog number: A0528)
l. CuSO4·5H2O (Duchefa Biochemie, catalog number: C0508)
m. FeNaEDTA (Duchefa Biochemie, catalog number: E0509)
n. FeSO4·7H2O (Duchefa Biochemie, catalog number: F0512)
o. H3BO3 (Duchefa Biochemie, catalog number: B0503)
p. MnSO·H2O (Duchefa Biochemie, catalog number: M0514)
q. Na2MO4·2H2O (Duchefa Biochemie, catalog number: S0525)
r. Na2EDTA·2H2O (Duchefa Biochemie, catalog number: E0511)
s. ZnSO4·7H2O (Duchefa Biochemie, catalog number: Z0526)
t. KI (Duchefa Biochemie, catalog number: P0518)
u. CoCl2·6H2O (Duchefa Biochemie, catalog number: C0507)
v. Sucrose (Duchefa Biochemie, catalog number: S0809)
w. D-Mannitol (Duchefa Biochemie, catalog number: M0803)
x. D-Glucose monohydrate (Duchefa Biochemie, catalog number: G0802)
y. Polyethylene glycol 4000 (PEG 4000) (Duchefa Biochemie, catalog number: P0804)
z. 2-(N-morpholino) ethane sulfonic acid (Duchefa Biochemie, catalog number: M1503)
aa. Biotin (Duchefa Biochemie, catalog number: B0603)
bb. Folic acid (Duchefa Biochemie, catalog number: F0608)
cc. Myo-inositol (Duchefa Biochemie, catalog number: I0609)
dd. Glycine (Duchefa Biochemie, catalog number: G0709)
ee. Nicotinic acid (Duchefa Biochemie, catalog number: N0611)
ff. Pyridoxine HCl (Duchefa Biochemie, catalog number: P0612)
gg. Thiamine HCl (Duchefa Biochemie, catalog number: T0614)
hh. D-pantothenate calcium (vitamin B5 calcium) (Duchefa Biochemie, catalog number: C0604)
ii. L-glutamic acid (Duchefa Biochemie, catalog number: G0707)
jj. L-phenylalanine (Duchefa Biochemie, catalog number: P0716)
kk. TopVision agarose (Thermo Scientific, Thermo Fisher Scientific, catalog number: R0491)
ll. 100 bp DNA ladder (Life Technologies, Thermo Fisher Scientific, catalog number: SM0241)
mm. Syber® safe DNA gel stain (Invitrogen, Thermo Fisher Scientific, catalog number: S33102)
nn. TriTrack DNA loading dye 6× (Life Technologies, Thermo Fisher Scientific, catalog number: R1161)
oo. Acetocarmine staining (Sigma-Aldrich, catalog number: 280370)
pp. NaClO (Sigma-Aldrich, catalog number: 1056142500)
qq. Tween-20 (Sigma-Aldrich, catalog number: P1379)
8. Supplements
a. Casein hydrolysate (Duchefa Biochemie, catalog number: C1301)
b. Activated charcoal (Sigma-Aldrich, catalog number: 31616)
Solutions
1. Macronutrients
a. NN macronutrients 10× (see Recipes)
b. MS macronutrients 10× (see Recipes)
c. C2D macronutrients 10× (see Recipes)
2. Micronutrients
a. NN micronutrients 100× (see Recipes)
b. MS micronutrients 1,000× (without FeEDTA) (see Recipes)
c. MS micronutrients 100× (see Recipes)
d. C2D micronutrients 1,000× (see Recipes)
3. Hormones
a. 2,4-Dichlorophenoxyacetic acid 1,000 µM (see Recipes)
b. 6-Benzylaminopurine 1,000 µM (see Recipes)
c. 1-Naphthaleneacetic acid 1,000 µM (see Recipes)
d. β-Naphthoxyacetic acid 1,000 µM (see Recipes)
4. Vitamins
a. NN vitamins 500× (see Recipes)
b. MS vitamins 500× (see Recipes)
c. C2D vitamins 1,000× (see Recipes)
d. B5 vitamins 500× (see Recipes)
e. Vitamins mix C1 500× (see Recipes)
f. Vitamins T 1,000×: (see Recipes)
5. Amino acids
a. Amino acids mix 1,000× (see Recipes)
6. Chemicals
a. FeEDTA 200× (see Recipes)
b. KCl 500 mM (see Recipes)
c. 2-(N-morpholino) ethanesulfonic acid 100 mM, pH 5.7 (see Recipes)
d. CaCl2·2H2O 1 M (see Recipes)
e. MgCl2·6H2O 500 mM (see Recipes)
f. NaCl 1 M (see Recipes)
7. Organics
a. Mannitol 1 M (see Recipes)
b. Glucose 3 M (see Recipes)
8. Culture media for induction of embryogenic calli (see Recipes)
a. NB2 (culture medium for induction of embryogenic calli from in vitro leaves)
b. PIV (culture medium for induction of embryogenic calli from stamens and pistils)
c. MSII (culture medium for induction of embryogenic calli from stamens and pistils)
9. Culture medium for long-term maintenance of embryogenic calli (C1P) (see Recipes)
10. Culture medium for somatic embryos full germination (see Recipes)
11. Culture media for somatic embryos shooting (see Recipes)
a. C2D
b. C2D4B
c. MG1
d. MG1–10B
12. Culture media for full plant development and rooting (see Recipes)
a. RIM
b. MSN
13. Culture media for protoplasts cultivation (see Recipes)
a. Solid culture medium
b. Liquid culture medium
14. Solution for protoplast isolation, purification, and transfection (see Recipes)
a. Digestion solution
b. Digestion solution without enzymes
c. Wash solution
d. W5 solution
e. MMG solution
f. PEG solution
Recipes
1. Macronutrients
a. NN macronutrients 10×: 2.2 g/L CaCl2·2H2O, 9.5 g/L KNO3, 7.2 g/L NH4NO3, 1.85 g/L MgSO4·7H2O, 0.68 g/L KH2PO4
b. MS macronutrients 10×: 16.5 g/L of NH4NO3, 4.4 g/L of CaCl2·2H2O, 3.7 g/L of MgSO4·7H2O, 19.7 g/L of KNO3 and 1.7 g/L of KH2РО4
c. C2D macronutrients 10×: 16.5 g/L NH4NO3, 19 g/L KNO3, 3.7 g/L MgSO4·7H2O, 1.7 g/L KH2PO4, 7.1 g/L Ca(NO3)2·4H2O, 0.28 g/L FeSO4·7H2O, 0.37 g/L Na2EDTA
2. Micronutrients
a. NN micronutrients 100×: 2.5 mg/L CuSO4·5H2O, 3.67 g/L FeNaEDTA, 1 g/L H3BO3, 1.9 g/L MnSO4·H2O, 25 mg/L Na2MoO4·2H2O, 1g/L ZnSO4·7H2O
b. MS micronutrients 1,000× (without FeEDTA): 6.2 g/L H3BO3, 16.9 g/L MnSO4·H2O, 8.6 g/L ZnSO4·7H2O, 0.83 g/L KI, 0.25 g/L Na2MoO4·2H2O, 25 mg/L CuSO4·5H2O, 25 mg/L CoCl2·6H2O
c. MS micronutrients 100×: 0.62 g/L H3BO3, 3.67 g/L FeNaEDTA, 1.69 g/L of MnSO4·H2O, 0.86 g/L ZnSO4·7H2O, 0.083 g/L of KI, 0.025 g/L Na2MoO4·2H2O, 0.0025 g/L CuSO4·5H2O, 0.0025 g/L CoCl2·6H2O
d. C2D micronutrients 1,000×: 0.64 g/L MnSO4·H2O, 6.2 g/L H3BO3, 8.6 g/L ZnSO4·7H2O, 0.25 g/L Na2MoO4·2H2O, 25 mg/L CuSO4·5H2O, 25 mg/L CoCl2·6H2O
3. Hormones
Note: All the following hormone powders are soluble in NaOH 1 M. It is important to add NaOH 1 M to the weighed powder before the addition of water.
a. 2,4-Dichlorophenoxyacetic acid 1,000 µM: 0,221 g/L of 2.4-dichlorophenoxyacetic acid
b. 6-Benzylaminopurine 1,000 µM: 0.2252 g/L of 6-benzylaminopurine
c. 1-Naphthaleneacetic acid 1,000 µM: 0.1862 g/L of 1-naphthaleneacetic acid
d. β-Naphthoxyacetic acid 1,000 µM: 0.2022 g/L of β-naphthoxyacetic acid
4.Vitamins
a. NN vitamins 500×: 25 mg/L biotin, 0.25 g/L folic acid, 1 g/L glycine, 50 g/L myo-Inositol, 2.5 g/L nicotinic acid, 0.25 g/L Pyridoxine HCl, 0.25 g/L thiamine HCl
b. MS vitamins 500×: 1 g/L glycine, 50 g/L myo-inositol, 0.25 g/L nicotinic acid, 0.25 g/L pyridoxine HCl, 0.05 g/L thiamine HCl
c. C2D vitamins 1,000×: 1 g/L thiamine HCl, 10 g/L myo-inositol, 1 g/L nicotinic acid, 1 g/L pyridoxine HCl
d. B5 vitamins 500×: 50 g/L myo-inositol, 5 g/L thiamine HCl, 0.5 g/L nicotinic acid, 0.5 g/L pyridoxine HCl
e. Vitamins mix C1 500×: 50 g/L of myo-inositol, 5 g/L of nicotinic acid, 5 g/L of thiamine-HCI, 0.5 g/L of pyridoxine-HCl, 0.5 g/L of calcium pantothenate, 0.005 g/L of biotin
f. Vitamins T 1,000×: 50 g/L myo-inositol, 1 g/L nicotinic acid, 1 g/L thiamine HCl, 1 g/L pyridoxine HC, 1 g/L calcium pantothenate, 0.01 g/L biotin.
5. Amino acids
a. Amino acids mix 1,000×: 100 g/L L-glutamic acid (monosodium salt), 10 g/L L-phenylalanine, 2 g/L glycine
6. Chemicals
a. FeEDTA 200×: 7.44 g/L of Na2EDTA·2H2O, 1.86 g/L of FeSO4·7H2O
b. KCl 500 mM: 37.775 g/L of KCL
c. 2-(N-morpholino) ethanesulfonic acid 100 mM, pH 5.7: 19.52 g/L of 2-(N-morpholino) ethanesulfonic acid.
Note: Adjust the pH of this solution to 5.7 with KOH 1 M
d. CaCl2·2H2O 1 M: 146.9 g/L of CaCl2·2H2O
e. MgCl2·6H2O 500 mM: 101.655 g/L of MgCl2·6H2O
f. NaCl 1 M: 58.44 g/L of NaCl
7. Organics
a. Mannitol 1 M: 182.17 g/L of D-mannitol
b. Glucose 3 M: 594.6 g/L of glucose
8. Culture media for induction of embryogenic calli
a. NB2 (culture medium for induction of embryogenic calli from in vitro leaves)
| Component | Stock concentration | Final concentration | Quantity or Volume (1 L) |
|---|---|---|---|
| NN macronutrients | 10× | 1× | 100 mL |
| NN micronutrients | 100× | 1× | 10 mL |
| MS vitamins | 500× | 1× | 2 mL |
| 6-benzylaminopurine | 1,000 μM | 1.0 μM | 1 mL |
| 2,4-dichlorophenoxyacetic acid | 1,000 μM | 5.0 μM | 5 mL |
| Myo-inositol | / | 0.1 g/L | 0.1 g |
| Sucrose | / | 20 g/L | 20 g |
| Adjust to final pH 6.0 with KOH 1 M | |||
| Agar TC | / | 7 g/L | 7 g |
b. PIV (culture medium for induction of embryogenic calli from stamens and pistils)
| Component | Stock concentration | Final concentration | Quantity or Volume (1 L) |
|---|---|---|---|
| NN macronutrients | 10× | 1× | 100 mL |
| MS micronutrients | 1,000× | 1× | 1 mL |
| FeEDTA | 200× | 1× | 5 mL |
| B5 vitamins | 500× | 1× | 2 mL |
| 6-benzylaminopurine | 1,000 μM | 8.9 μM | 8.9 mL |
| 2,4-dichlorophenoxyacetic acid | 1,000 μM | 4.5 μM | 4.5 mL |
| Sucrose | 60 g/L | 60 g | |
| Adjust to final pH 5.7 with KOH 1 M | |||
| Phytagel | / | 3 g/L | 3 g |
c. MSII (culture medium for induction of embryogenic calli from stamens and pistils)
| Component | Stock concentration | Final concentration | Quantity or Volume (1 L) |
|---|---|---|---|
| MS macronutrients | 10× | 1× | 100 mL |
| MS micronutrients | 100× | 1× | 10 mL |
| MS vitamins | 500× | 1× | 2 mL |
| β-naphthoxyacetic acid | 1,000 μM | 2.5 μM | 2.5 mL |
| 6-benzylaminopurine | 1,000 μM | 5 μM | 5 mL |
| 2,4-dichlorophenoxyacetic acid | 1,000 μM | 2.5 μM | 2.5 mL |
| Myo-inositol | / | 0.1 g/L | 0.1 g |
| Sucrose | / | 20 g/L | 20 g |
| Adjust to final pH 6.0 with KOH 1 M | |||
| Agar TC | / | 7 g/L | 7 g |
9. Culture medium for long-term maintenance of embryogenic calli (C1P)
| Component | Stock concentration | Final concentration | Quantity or Volume (1 L) |
|---|---|---|---|
| MS macronutrients | 10× | 1× | 100 mL |
| MS micronutrients | 1,000× | 1× | 1 mL |
| Vitamins C1 | 500× | 1× | 2 mL |
| AA mix | 1,000× | 1× | 1 mL |
| Fe-EDTA | 200× | 1× | 5 mL |
| 2,4-dichlorophenoxyacetic acid | 1,000 μM | 5 μM | 5 mL |
| 6-benzylaminopurine | 1,000 μM | 1 μM | 1 mL |
| Casein hydrolysate | / | 1 g/L | 1 g |
| Sucrose | / | 30 g/L | 30 g |
| Adjust to final pH 5.8 with KOH 1 M | |||
| Phytagel | / | 5g/L | 5 g |
10. Culture medium for somatic embryos full germination
| Component | Stock concentration | Final concentration | Quantity or Volume (1 L) |
|---|---|---|---|
| NN macronutrients | 10× | 1× | 100 mL |
| NN micronutrients | 100× | 1× | 10 mL |
| NN vitamins | 500× | 1× | 2 mL |
| Sucrose | / | 30 g/L | 30 g |
| Adjust to final pH 5.8 with KOH 1 M | |||
| Gelrite | / | 2 g/L | 2 g |
11. Culture media for somatic embryo shooting
a. C2D
| Component | Stock concentration | Final concentration | Quantity or Volume (1 L) |
|---|---|---|---|
| C2D macronutrients | 10× | 1× | 100 mL |
| C2D micronutrients | 1,000× | 1× | 1 mL |
| C2D vitamins | 1,000× | 1× | 1 mL |
| Sucrose | / | 30 g/L | 30 g |
| Adjust to final pH 5.8 with KOH 1 M | |||
| Agar TC | / | 7 g/L | 7 g |
b. C2D4B
| Component | Stock concentration | Final concentration | Quantity or Volume (1 L) |
|---|---|---|---|
| C2D macronutrients | 10× | 1× | 100 mL |
| C2D micronutrients | 1,000× | 1× | 1 mL |
| C2D vitamins | 1,000× | 1× | 1 mL |
| 6-benzylaminopurine | 1,000 μM | 4 μM | 4 mL |
| Sucrose | / | 30 g/L | 30 g |
| Adjust to final pH 5.8 with KOH 1 M | |||
| Agar TC | / | 7 g/L | 7 g |
c. MG1
| Component | Stock concentration | Final concentration | Quantity or Volume (1 L) |
|---|---|---|---|
| NN macronutrients | 10× | 1× | 100 mL |
| MS micronutrients | 1,000× | 1× | 1 mL |
| Fe-EDTA | 200× | 1× | 5 mL |
| B5 vitamins | 500× | 1× | 2 mL |
| Sucrose | / | 30 g/L | 30 g |
| Adjust to final pH 5.8 with KOH 1 M | |||
| Agar TC | / | 7 g/L | 7 g |
| Activated charcoal | / | 2.5 g/L | 2.5 g |
d. MG1-10B
| Component | Stock concentration | Final concentration | Quantity or Volume (1 L) |
|---|---|---|---|
| NN macronutrients | 10× | 1× | 100 mL |
| MS micronutrients | 1,000× | 1× | 1 mL |
| Fe-EDTA | 200× | 1× | 5 mL |
| B5 vitamins | 500× | 1× | 2 mL |
| 6-benzylaminopurine | 1,000 μM | 10 μM | 10 mL |
| Sucrose | / | 30 g/L | 30 g |
| Adjust to final pH = 5.8 with KOH 1 M | |||
| Agar TC | / | 7 g/L | 7 g |
| Activated charcoal | / | 2.5 g/L | 2.5 g |
12. Culture media for full plant development and rooting
a. RIM
| Component | Stock concentration | Final concentration | Quantity or Volume (1 L) |
|---|---|---|---|
| MS macronutrients | 10× | 1× | 100 mL |
| MS micronutrients | 1,000× | 1× | 1 mL |
| Fe-EDTA | 200× | 1× | 5 mL |
| Vitamins T | 1,000× | 1× | 1 mL |
| 1-naphthaleneacetic acid | 1,000 μM | 0.5 μM | 500 μL |
| Sucrose | / | 30 g/L | 30 g |
| Adjust to final pH 6.0 with KOH 1 M | |||
| Agar TC | / | 7 g/L | 7 g |
b. MSN
| Component | Stock concentration | Final concentration | Quantity or Volume (1 L) |
|---|---|---|---|
| MS macronutrients | 10× | 1× | 100 mL |
| MS micronutrients | 100× | 1× | 10 mL |
| MS vitamins | 500× | 1× | 2 mL |
| 1-naphthaleneacetic acid | 1,000 μM | 0.5 μM | 500 μL |
| Sucrose | / | 30 g/L | 30 g |
| Adjust to final pH 5.8 with KOH 1 M | |||
| Agar TC | / | 7 g/L | 7 g |
13. Culture media for protoplast cultivation
a. Solid culture medium
| Component | Stock concentration | Final concentration | Quantity or Volume (50 mL) |
|---|---|---|---|
| NN macronutrients | 10× | 1× | 5 mL |
| NN micronutrients | 100× | 1× | 500 μL |
| NN vitamins | 500× | 1× | 100 μL |
| 1-naphthaleneacetic acid | 1,000 μM | 10 μM | 500 μL |
| 6-benzylaminopurine | 1,000 μM | 2 μM | 100 μL |
| Glucose | 3 M | 0.3 M | 5 mL |
| Sucrose | / | 30 g/L | 1.50 g |
| Adjust to final pH 5.7 with KOH 1 M | |||
| Gelrite | / | 2 g/L | 0.1 g |
b. Liquid culture medium
| Component | Stock concentration | Final concentration | Quantity or Volume (50 mL) |
|---|---|---|---|
| NN macronutrients | 10× | 1× | 5 mL |
| NN micronutrients | 100× | 1× | 500 μL |
| NN vitamins | 500× | 1× | 100 μL |
| 1-Naphthaleneacetic acid | 1,000 μM | 10 μM | 500 μL |
| 6-benzylaminopurine | 1,000 μM | 2 μM | 100 μL |
| Glucose | 3 M | 0.3 M | 5 mL |
| Sucrose | / | 30 g/L | 1.50 g |
| Adjust to final pH 5.7 with KOH 1 M | |||
| Activated charcoal | / | 3 g/L | 0.15 g |
14. Solution for protoplast isolation, purification, and transfection
a. Digestion solution
| Component | Stock concentration | Final concentration | Quantity or Volume (20 mL) |
|---|---|---|---|
| Cellulase R10 | / | 2% w/v | 0.4g |
| Macerozyme R10 | / | 1 % w/v | 0.2 g |
| Pectolyase Y-23 | / | 0.05 % w/v | 0.01 g |
| CaCl2·2H2O | 1 M | 10 mM | 200 μL |
| 2-(N-morpholino) ethanesulfonic acid, pH 5.7 | 100 mM | 5 mM | 1 mL |
| Mannitol | 1 M | 0.5 M | 10 mL |
| Adjust to final pH 5.7 with KOH 1 M |
b. Digestion solution without enzymes
| Component | Stock concentration | Final concentration | Quantity or Volume (20 mL) |
|---|---|---|---|
| CaCl2·2H2O | 1 M | 10 mM | 200 μL |
| 2-(N-morpholino) ethanesulfonic acid, pH 5.7 | 100 mM | 5 mM | 1 mL |
| Mannitol | 1 M | 0.5 M | 10 mL |
| Adjust to final pH 5.7 with KOH 1 M |
c. Wash solution
| Component | Stock concentration | Final concentration | Quantity or Volume (50 mL) |
|---|---|---|---|
| CaCl2·2H2O | 1 M | 10 mM | 500 μL |
| Mannitol | 1 M | 0.5 M | 25 mL |
| Adjust to final pH 5.7 with KOH 1 M |
d. W5 solution
| Component | Stock concentration | Final concentration | Quantity or Volume (40 mL) |
|---|---|---|---|
| CaCl2·2H2O | 1 M | 125 mM | 5 mL |
| 2-(N-morpholino) ethanesulfonic acid, pH 5.7 | 100 mM | 2 mM | 800 μL |
| NaCl | 1 M | 154 mM | 6.16 mL |
| KCl | 500 mM | 5 mM | 400 μL |
e. MMG solution
| Component | Stock concentration | Final concentration | Quantity or Volume (10 mL) |
|---|---|---|---|
| Mannitol | 1 M | 0.4 M | 4 mL |
| MgCl2·6H2O | 500 mM | 15 mM | 300 μL |
| 2-(N-morpholino) ethanesulfonic acid, pH 5.7 | 100 mM | 4 mM | 400 μL |
f. PEG solution
| Component | Stock concentration | Final concentration | Quantity or Volume (10 mL) |
|---|---|---|---|
| Mannitol | 1 M | 0.2 M | 2 mL |
| CaCl2·2H2O | 500 mM | 100 mM | 1 mL |
| PEG 4000 | / | 40% | 4 g |
Laboratory supplies
1. Steri vent container 107 × 94 × 96 mm (Duchefa Biochemie, catalog number: S1682)
2. Nylon filter 60 μM (AGRINOVA)
3. Petri dishes 92 × 16 (VWR, catalog number: 391-0493)
4. Petri dishes 60 × 15 (Greiner, catalog number: 628161)
5. Petri dishes 35 × 15 (Thermo Scientific, catalog number: 153066)
6. Sterile needles 0.7 × 50 mm (Henke Sass Wolf, catalog number: 471,0007050)
7. Sterile syringes 1 mL (Terumo, catalog number: MDSS01SE)
8. Sterile syringes 50 mL (NIPRO, catalog number: BSS131)
9. Sterile syringes 20 mL (BD Plastipak, catalog number: 300613)
10. Sterile centrifuge tubes, Falcon 15 mL (VWR, catalog number: VWRI525-0607)
11. Sterile centrifuge tubes, Falcon 50 mL (VWR, catalog number: VWRI525-0612)
12. Pipettes (Eppendorf)
13. Fast-Read102® (Kova International, catalog number: BVS100H)
14. Filter 0.2 μm (Sarstedt, catalog number: 83.1826.001)
15. Pasteur 3 mL (Sarstedt, catalog number: D-51588)
16. Filter tips (2,5 μL, 20 μL, 200 μL, 1,000 μL, 5 mL)
17. Scalpel blades 21 and 11 (Duchefa)
18. Microscope slides
19. Coverslips
20. PCR tubes
21. 1.5 mL Eppendorf tubes
22. Sterile scalpels
23. Sterile forceps
24. Parafilm
Equipment
1. Heating magnetic stirrer (VWR®, model: 442-0664)
2. pH meter (CRISON, model: BASIC 20+)
3. Weighing balance (OHAUS, model: AX422/E)
4. Analytical balance (OHAUS, model: PA114C)
5. Glass microsphere sterilizer (AgnTho’s AB, model: steri 250, Art-Nr 31’101)
6. ChemiDoc imaging system (Bio-Rad, model: 12003153)
7. Thermal cycler (Bio-Rad, model: S1,000)
8. Horizontal laminar flow cabinet (BIOAIR, model: aura HZ72)
9. PowerPacTM basic power supply (Bio-Rad, model: 1645050)
10. Horizontal electrophoresis cells (Bio-Rad, model: Sub-Cell GT)
11. Horizontal electrophoresis cells (ELETTROFOR, models: OA-50; OA-78)
12. INCU-Line 150R (VWR®, model: 390-1338)
13. Benchtop microcentrifuge (Eppendorf, catalog number: 5420)
14. Centrifuge (Eppendorf, model: 5804 R)
15. Stereomicroscopes (Leica, models: MZ16 F; EZ4)
16. Optical microscope (Leica, model: DM2500)
Procedure
文章信息
稿件历史记录
提交日期: Jul 19, 2024
接收日期: Dec 4, 2024
在线发布日期: Jan 9, 2025
出版日期: Feb 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Bertini, E., D’Incà, E., Zattoni, S., Lissandrini, S., Cattaneo, L., Ciffolillo, C., Amato, A., Fasoli, M. and Zenoni, S. (2025). Transgene-free Genome Editing in Grapevine. Bio-protocol 15(4): e5190. DOI: 10.21769/BioProtoc.5190.
分类
植物科学 > 植物分子生物学 > 遗传分析
植物科学 > 植物细胞生物学 > 细胞分离
生物科学 > 生物技术 > CRISPR/Cas9
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link