- EN - English
- CN - 中文
Closed Systems to Study Plant–Filamentous Fungi Associations: Emphasis on Microscopic Analyses
研究植物与丝状真菌互作的闭合系统:重点关注显微分析
发布: 2025年02月20日第15卷第4期 DOI: 10.21769/BioProtoc.5186 浏览次数: 2849
评审: Shweta PanchalAnonymous reviewer(s)

相关实验方案
基于 Emu 的可重复计算流程:在高性能计算集群上实现高保真土壤—植物微生物组解析
Henrique M. Dias [...] Christopher Graham
2026年01月20日 370 阅读
Abstract
In nature, filamentous fungi interact with plants. These fungi are characterized by rapid growth in numerous substrates and under minimal nutrient requirements. Investigating the interaction of these fungi with their plant hosts under controlled conditions is of importance for many researchers aiming to proceed with molecular or microscopical investigations of their favorite plant–fungus interaction system. The speed of growth of these fungi complicates transferring plant–fungal interaction systems in laboratory conditions. The issue is more complicated when monoxenic conditions are desired, to ensure that only two members (a fungus and a plant) are present in the system under study. Here, two simple closed systems for investigating plant–filamentous fungi associations under laboratory, monoxenic conditions are described, along with their limitations. The plant and fungal growth conditions, methods for sampling, staining, sectioning, and subsequent microscopical imaging of colonized plant tissues with affordable, common laboratory tools are described.
Key features
• Setting up closed systems for microscopical observations of plant–filamentous fungi (emphasis on model legumes–Fusaria) associations and temporal in vivo observations of the association(s).
• Preparing root samples for microscopical observations: staining, sectioning, and mounting on microscopical slides.
• Using low-cost equipment for performing microscopical observations and imaging.
• Using fluorescence microscopy: provision of common fluorophores to highlight specific plant and fungal tissues, compartments, and structures.
Keywords: Plant–microbe interactions (植物-微生物互作)Graphical overview
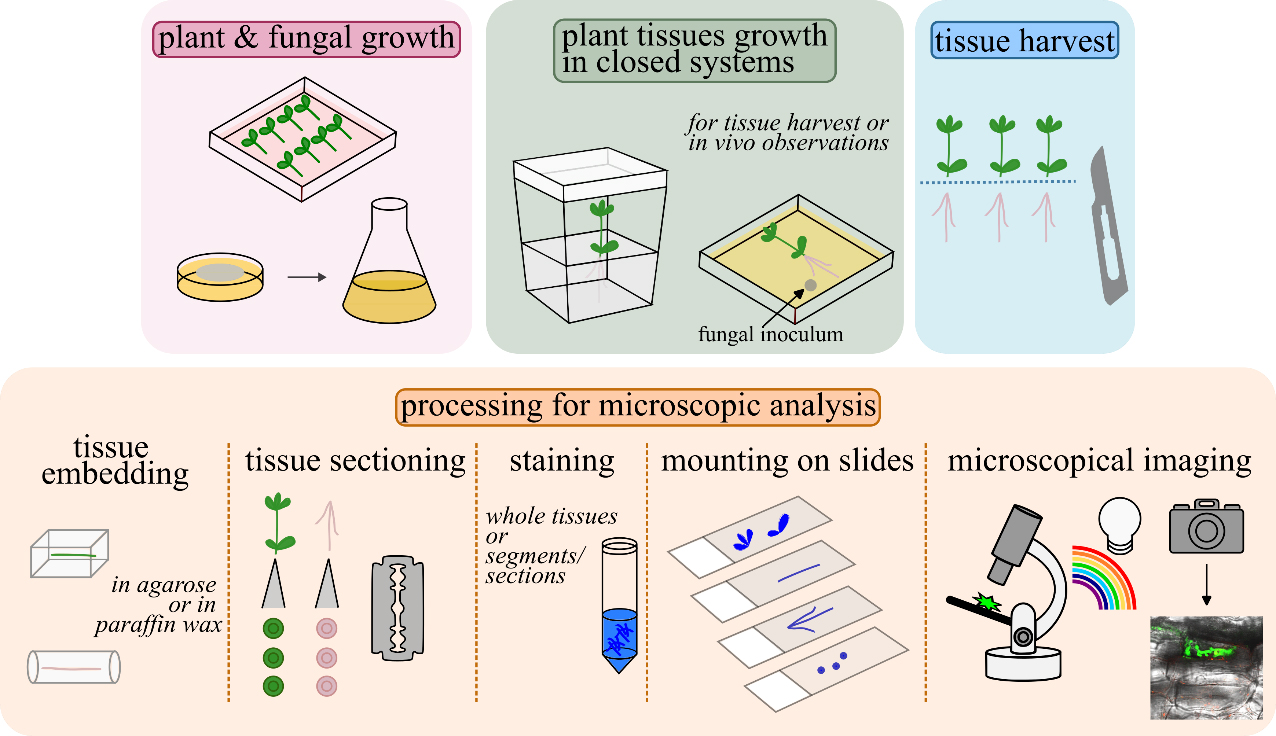
Graphical overview of closed systems used to study plant–filamentous fungi associations and subsequent tissue processing for microscopic analyses. Lotus japonicus seeds are scarified, decontaminated, and germinated in Petri dishes. A Fusarium strain of choice is grown to collect propagules for plant inoculations. L. japonicus plantlets are transplanted in magenta boxes/glass jars or in square Petri dishes and inoculated with the fungus. Square Petri dishes are recommended for in vivo observations. For further tissue(s) processing, root/stem/leaf tissues are collected, embedded in agarose or in paraffin wax, and sectioned by hand, in cases where sectioning is necessary. Either whole root/stem/leaf tissues or tissue sections are stained to highlight inter- and/or intracellular fungal structures. Stained tissues are mounted on slides and observed using a compound microscope. An epifluorescence microscope is used when tissues are stained with fluorescent dyes to highlight specific plant tissues and compartments, fungal cells, and structures. A confocal system is used, if accessible, for sharper images and/or for live cell imaging.
Background
Plant–microbe interactions (PMIs) are of fundamental importance and occur everywhere in nature. Multiple microbial partners interact with root tissues and some of them will simultaneously colonize plant tissues. PMI research greatly benefits from the ability to grow plants in axenic (sterile) conditions [1]. Even in simplified systems, difficulties arise in distinguishing the partner responsible for a given response. Investigating individual, simplified, bipartite associations of the plant with a single microbial partner aids in understanding the reciprocal impact and deciphering specific mechanisms of response. Transferring PMIs in controlled laboratory conditions is of importance, especially when basic biological questions either related to molecular or cellular biology are to be answered. To perform and investigate PMIs under gnotobiotic conditions, model systems are necessary. The construction of model systems where plant roots associate with one or more selected microbial species allows researchers to investigate interactions in simpler systems than those occurring in nature [2].
In monoxenic conditions, for example, a plant species associates with only one microbial partner. Several closed systems have been reported, including agar plates, which have been used extensively in mycorrhizal research [3]. Here, we focus on the interaction of model plants with filamentous fungi. When investigating plant–filamentous fungi associations at the molecular and cellular level, setting up a model, closed system where the interaction will occur is important. The adjustment of several parameters that depend on the organism under investigation and the research questions posed should be considered. In any given model system, further processing of infected plant tissues depends on the research aim. For many studies, determining the degree of root colonization by fungi is necessary, as correlations to benefits for the plants may be inferred, such as promotion of growth and seed yield as well as tolerance against biotic and abiotic stress, or even correlations to loss of benefits, in cases of fungal over-colonization. In this respect, powerful staining techniques are required to visualize and estimate fungal growth within the roots [4].
Sectioning of plant tissues is also considered when a more detailed analysis is required, such as in determining the exact location of fungal cells or the infected plant cells and tissues, as well as revealing alterations on the plant/fungal side. Besides conventional microscopic analysis, the use of fluorescent dyes is also sometimes necessary to highlight specific colorless plant or fungal compartments, tissues, and/or deposition of plant substances, such as lignin and suberin [5–7].
Here, we describe in detail two closed, monoxenic systems of model legume plants with filamentous fungi by taking advantage of the previously described model interaction system of the legume Lotus japonicus with the endophytic, beneficial fungal strain Fusarium solani strain K (FsK) [8–10]. We emphasize the use of affordable tools and materials for performing these experiments. Furthermore, we describe the processing of tissues, including harvest, staining, and sectioning to visualize inter- and intracellular fungal structures to perform basic microscopic analysis. Finally, we present common fluorophores that one may consider if a more detailed microscopic analysis, using fluorescence microscopy, is required.
Materials and reagents
Biological materials
1. L. japonicus seeds (ecotype Gifu)
2. Fusarium strain [such as F. solani strain K (FsK), F. oxysporum forma specialis (f. sp.) medicaginis (Fom), Fusarium oxysporum f. sp. lycopersici (Fol); and Fusarium oxysporum f. sp. radicis-lycopersici (Forl) (see General note 1)]
Reagents
1. Deionized water
2. Sulfuric acid (H2SO4) (Sigma-Aldrich, catalog number: 258105)
3. Potato Dextrose Broth (Condalab, catalog number: 1261)
4. Commercial bleach (non-concentrated, containing ~5% w/v sodium hypochlorite, generic)
5. Murashige and Skoog (MS) medium including vitamins (Duchefa Biochemie, catalog number: M022)
6. Bacteriological Agar (Condalab, catalog number: 1800)
7. Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888)
8. Potassium nitrate (KNO3) (Chem-Lab NV, catalog number: CL00.1164)
9. Calcium nitrate tetrahydrate [Ca(NO3)2·4H2O] (Chem-Lab NV, catalog number: CL00.0340)
10. Potassium dihydrogen phosphate (KH2PO4) (Chem-Lab NV, catalog number: CL00.1146)
11. Magnesium sulfate heptahydrate (MgSO4·7H2O) (Chem-Lab NV, catalog number: CL00.1324)
12. Boric acid (H3BO3) (Chem-Lab NV, catalog number: CL00.0216)
13. Manganese(II) chloride tetrahydrate (MnCl2·4H2O) (Chem-Lab NV, catalog number: CL00.1328)
14. Zinc sulfate heptahydrate (ZnSO4·7H2O) (Chem-Lab NV, catalog number: CL00.2629)
15. Copper sulfate pentahydrate (CuSO4·5H2O) (Chem-Lab NV, catalog number: CL00.1127)
16. Molybdic acid (MoO3·H2O) (Supelco, catalog number: 100400)
17. Potassium chloride (KCl) (Chem-Lab NV, catalog number: CL00.1133)
18. Sodium ferric ethylenediaminetetraacetic acid (NaFeEDTA) (Sigma-Aldrich, catalog number: E6760)
19. Potassium iodide (KI) (Chem-Lab, catalog number: CL00.1134)
20. Sodium molybdate dihydrate (Na2MoO4·2H2O) (Applichem, catalog number: 131701)
21. Glycine (Duchefa Biochemie, catalog number: G0709)
22. Thiamine hydrochloride (Duchefa Biochemie, catalog number: T0614)
23. Pyridoxine Hydrochloride (Duchefa Biochemie, catalog number: P0612)
24. Nicotinic acid (Duchefa Biochemie, catalog number: N0611)
25. Myo inositol (Duchefa Biochemie, catalog number: I0609)
26. Agarose (low melt, Carl Roth, catalog number: 6351)
27. Ethanol (PanReac Applichem, catalog number: 147194)
28. Potassium hydroxide (KOH) (pellets, Chem-Lab, catalog number: CL00.1135)
29. Hydrochloric acid (HCl) (37+% HCl, Chem-Lab, catalog number: CL00.0310)
30. Chlorazol black (CB) (Sigma-Aldrich, catalog number: C1144)
31. Glycerol (PanReac Applichem, catalog number: 131339)
32. Lactic acid (PanReac Applichem, catalog number: 141034)
33. Trypan blue (TB) (Sigma-Aldrich, catalog number: T6146)
34. Black ink (Sheaffer, catalog number: 94231)
35. Acetic acid (BioChemica, catalog number: 131008)
36. Polyvinyl acetate (PVA) (Carl Roth, catalog number: 9154)
37. Liquid nitrogen (to collect tissues for subsequent molecular analysis, e.g., gene expression) (generic)
38. Paraffin wax flakes (generic)
Solutions
1. Potato dextrose broth medium (PDB) (prepared as per purchased product instructions)
2. Commercial bleach (containing ~5% w/v sodium hypochlorite) solution (20% v/v) (see Recipes)
3. Half-strength Murashige and Skoog (MS) medium including vitamins (see Recipes)
4. Isotonic solution (NaCl 0.85% w/v) (see Recipes)
5. Hoagland medium (see Recipes)
6. M medium (see Recipes)
7. Low melting temperature agarose solution (0.4% w/v) (see Recipes)
8. Ethanol solution (70% v/v) (see Recipes)
9. KOH (10% w/v) (see Recipes)
10. HCl (1 N) (see Recipes)
11. CB stain solution (0.05% w/v in water:glycerol:lactic acid 1:1:1) (see Recipes)
12. TB stain solution (0.05% w/v in water:glycerol:lactic acid 1:1:1) (see recipes)
13. Black ink (5% v/v in 5% v/v acetic acid) (see Recipes)
14. Agarose solution (6%–8% w/v) (see Recipes)
15. Polyvinyl-Lacto-Glycerol (PVLG) (see Recipes)
16. Glycerol (50%–90% v/v) (see Recipes)
Recipes
1. Commercial bleach solution (20% v/v)
| Reagent | Final concentration | Quantity or Volume |
| Commercial bleach (containing ~5% w/v sodium hypochlorite) | 20% | 10 mL |
| H2O | n/a | 40 mL |
| Total | n/a | 50 mL |
2. Half-strength MS medium including vitamins [11]
| Reagent | Final concentration | Quantity or Volume |
| MS medium | 0.5× | 2.2 g |
| Agar | 1% | 10 g |
| Total | n/a | 1,000 mL |
Adjust the pH of the medium to 5.5 before sterilization. Sterilize at 121 °C for 15 min.
3. Isotonic solution (NaCl 0.85% w/v)
| Reagent | Final concentration | Quantity or Volume |
| NaCl | 0.85% | 0.85 g |
| Total | n/a | 100 mL |
Sterilize at 121 °C for 15 min.
4. Hoagland medium [12,13]
| Reagent | Final concentration | Quantity or Volume |
| KNO3 | 5 mM | 5 mL of 1 M |
| Ca(NO3)2·4H2O | 5 mM | 5 mL of 1 M |
| KH2PO4 | 1 mM | 1 mL of 1 M |
| MgSO4·7H2O | 2 mM | 2 mL of 1 M |
| FeEDTA | 1–5 mg/L | 1–5 mL of 1,000 mg/L |
| Micronutrient stock solution* | - | 1 mL |
| Total | n/a | 1,000 mL |
*Micronutrient stock solution for Hoagland medium
| Reagent | Final concentration | Quantity or Volume |
| H3BO3 | - | 2.86 g |
| MnCl2·4H2O | - | 1.81 g |
| ZnSO4·7H2O | - | 0.22 g |
| CuSO4·5H2O | - | 0.08 g |
| MoO3·H2O | - | 0.02 g |
| Total | n/a | 1,000 mL |
If necessary, sterilize the medium at 121 °C for 15 min.
5. M medium [14]
| Reagent | Final concentration | Quantity or Volume |
| MgSO4·7H2O | - | 731 mg |
| KNO3 | - | 80 mg |
| KCl | - | 65 mg |
| KH2PO4 | - | 4.8 mg |
| Ca(NO3)2·4H2O | - | 288 mg |
| NaFeEDTA | - | 8 mg |
| KI | - | 0.75 mg |
| MnCl2·4H2O | - | 6 mg |
| ZnSO4·7H2O | - | 2.65 mg |
| H3BO3 | - | 1.5 mg |
| CuSO4·5H2O | - | 0.13 mg |
| Na2MoO4·2H2O | - | 0.0024 mg |
| Glycine | - | 3 mg |
| Thiamine hydrochloride | - | 0.1 mg |
| Pyridoxine Hydrochloride | - | 0.1 mg |
| Nicotinic acid | - | 0.5 mg |
| Myo inositol | - | 50 mg |
| Agar | 0.4%–0.8% | 4–8 g |
| Total | n/a | 1,000 mL |
Adjust the pH of the medium to 5.5 before sterilization. Sterilize at 121 °C for 15 min.
6. Low melting temperature agarose solution (0.4% w/v)
| Reagent | Final concentration | Quantity or Volume |
| Agarose | 0.4% | 0.1 g |
| Total | n/a | 25 mL |
7. Ethanol solution (70% v/v)
| Reagent | Final concentration | Quantity or Volume |
| EtOH | 70% | 70 mL |
| Total | n/a | 100 mL |
8. KOH (10% w/v)
| Reagent | Final concentration | Quantity or Volume |
| KOH | 10% | 10 g |
| Total | n/a | 100 mL |
9. HCl (1 N)
| Reagent | Final concentration | Quantity or Volume |
| HCl (37% w/v, 12.08 N) | 1 N | 8.28 mL |
| Total | n/a | 100 mL |
10. CB stain solution (0.05% w/v in water:glycerol:lactic acid 1:1:1)
| Reagent | Final concentration | Quantity or Volume |
| Chlorazol black | 0.05% | 0.075 g |
| H2O | - | 50 mL |
| Glycerol | - | 50 mL |
| Lactic acid | - | 50 mL |
| Total | n/a | 150 mL |
11. TB stain solution (0.05% w/v in water:glycerol:lactic acid 1:1:1)
| Reagent | Final concentration | Quantity or Volume |
| Trypan blue | 0.05% | 0.075 g |
| H2O | - | 50 mL |
| Glycerol | - | 50 mL |
| Lactic acid | - | 50 mL |
| Total | n/a | 150 mL |
12. Black ink stain solution (5% v/v in 5% v/v acetic acid) [15]
| Reagent | Final concentration | Quantity or Volume |
| Black ink (Sheaffer) | 5% | 5 mL |
| Acetic acid | 5% | 5 mL |
| H2O | - | 90 mL |
| Total | n/a | 100 mL |
13. Agarose solution (6%–8% w/v)
| Reagent | Final concentration | Quantity or Volume |
| Agarose | 6%–8% | 6–8 g |
| Total | n/a | 100 mL |
14. PVLG
| Reagent | Final concentration | Quantity or Volume |
| Water | - | 100 mL |
| Lactic acid | - | 100 mL |
| Glycerol | - | 10 mL |
| PVA | - | 16.6 g |
| Total | n/a | 210 mL |
15. Glycerol (50%–90% v/v)
| Reagent | Final concentration | Quantity or Volume |
| Glycerol | 50%–90% | 15–27 mL |
| Total | n/a | 30 mL |
Laboratory supplies
1. Eppendorf tubes (1.5 and 2.0 mL) (D. Dutscher, catalog numbers: 033511 and 033297)
2. Glass Pasteur pipette(s) (D. Dutscher, catalog number: 251564)
3. Rubber teat (pipetting aid for Pasteur pipettes) (Sigma-Aldrich, catalog number: Z111597)
4. Pipette tips (Kisker, catalog numbers: VL700G and VL004G for 1-200 and 100-1000μl, respectively)
5. Pipette(s) (Gilson, catalog number: F144058M and F144059M for Pipetman P200 and P1000, respectively)
6. Forceps (generic)
7. Petri dishes (94/16 mm, Greiner Bio-One, catalog number: 633181)
8. Square Petri dishes (120/120/17 mm, Greiner Bio-One, catalog number: 688102)
9. Aluminum foil (generic)
10. Scalpel (generic)
11. Parafilm (D. Dutscher, catalog number: 090260)
12. Sterile cheesecloth (generic)
13. Sterile glass funnel (Boro Simax)
14. Sterile Falcon tubes (Sarstedt, catalog number: 62.547.254)
15. Neubauer chamber (hemacytometer) (Marienfeld Superior, catalog number: 0640030)
16. Cover glass for Neubauer chamber (20x26mm, Marienfeld Superior, catalog number: 0350000)
17. Magenta boxes (Phyta Jar, 74 × 71 × 98 mm, HIMEDIA, catalog number: PW1138-50NO), or
18. Glass jars with lid (~370 mL) (generic)
19. Substrate (sand:vermiculite mixture, 2:1) (generic)
20. Injection syringe & needle (generic)
21. Dissecting (inoculation) needle (generic)
22. Gas permeable plastic film (25-μm) (Film 25, Lumox, Sarstedt, catalog number: 94.6077.316)
23. Filter paper (generic)
24. Small plastic zip bags, Falcon tubes, or similar item(s) for tissue storage
25. Small strainer (generic)
26. Razor blades (generic)
27. Plastic Pasteur pipette (with cut end) (Biosigma, catalog number: BSV136)
28. Conical flask or beaker (Boro Simax)
29. Silicone rubber flat embedding mold (Agar Scientific, AGG3530), or
30. Molds made of aluminum foil
31. Microscope slides (Knittel, 76x26mm, catalog number: 303160)
32. Coverslips (Knittel, 24x50mm, catalog number: VD12450Y1A.01)
33. Nut (generic)
34. Bolt (~2 cm in depth) (generic)
35. Fine point forceps, or brush (generic)
36. Gloves (generic)
37. Lab coat (generic)
Equipment
1. Tube vortex mixer (Velp Scientifica)
2. Fume hood (Equip LaboTM Fume Hood)
3. Laminar flow hood (Telstar, Bio II Advance Plus 4)
4. Shaking incubator (Labtech)
5. Autoclave (Raypa, AES-50)
6. Refrigerator (4 °C) (generic)
7. Plant growth chamber (grow tent for indoor cultivation of plants, custom-made)
8. Centrifuge (Hermle)
9. Water bath (JP Selecta)
10. Hot plate stirrer (for agarose melting) (Agimatic-E)
11. Stereoscope (Novex Holland)
12. Oven (JP Selecta)
13. Compound light microscope (Novex Holland)
14. Fluorescence microscope (Olympus BX53 upright microscope)
15. Confocal microscope system (Zeiss LSM800 system)
16. Camera for imaging (Olympus DP74)
Software and datasets
1. Appropriate software for image acquisition (here, the cellSens imaging software was used)
Procedure
文章信息
稿件历史记录
提交日期: Sep 12, 2024
接收日期: Nov 21, 2024
在线发布日期: Jan 5, 2025
出版日期: Feb 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Skiada, V. and Papadopoulou, K. K. (2025). Closed Systems to Study Plant–Filamentous Fungi Associations: Emphasis on Microscopic Analyses. Bio-protocol 15(4): e5186. DOI: 10.21769/BioProtoc.5186.
分类
植物科学 > 植物细胞生物学 > 组织分析
植物科学 > 植物免疫 > 宿主-细菌相互作用
细胞生物学 > 组织分析 > 组织成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link