- EN - English
- CN - 中文
A Protocol to Purify Human Mediator Complex From Freestyle 293-F Cells
从FreeStyle 293-F细胞中纯化人类介导因子复合物的实验方案
发布: 2025年02月20日第15卷第4期 DOI: 10.21769/BioProtoc.5185 浏览次数: 2145
评审: Joana Alexandra Costa ReisRohini Ravindran NairAnonymous reviewer(s)

相关实验方案

适用于 LC–MS/MS 蛋白质组学分析的大体积细胞培养上清分泌组样品制备方法优化
Basil Baby Mattamana [...] Peter Allen Faull
2025年12月20日 1210 阅读
Abstract
The Mediator, a multi-subunit protein complex in all eukaryotes, comprises the core mediator (cMED) and the CDK8 kinase module (CKM). As a molecular bridge between transcription factors (TFs) and RNA polymerase II (Pol II), the Mediator plays a critical role in regulating Pol II–dependent transcription. Considering its large size and complex composition, conducting in vitro studies on the Mediator complex is challenging, especially when isolating the intact and homogeneous complex from human cells. Here, we present a method to purify the intact CKM-cMED complex from FreeStyle 293-F cells (293-F cells), which offers advantages for performing large-scale protein purification. To isolate the CKM-bound cMED without the presence of Pol II, FLAG-tagged CDK8, a subunit of the CKM complex, was expressed in 293-F cells for purification, as CKM and Pol II are mutually exclusive in their interaction with cMED. The complex is isolated from nuclear extracts through immunoaffinity purification and further purified by glycerol gradient to enhance its homogeneity. This protocol provides a time- and cost-efficient way to purify the endogenous Mediator complex for structural- and functional-based studies.
Key features
• This protocol describes a method for purifying the endogenous Mediator complex, free of Pol II, from 293-F cells.
• Does not require the use of crosslinkers, offering advantages for structural and functional studies.
Keywords: Mediator (介导因子)Graphical overview
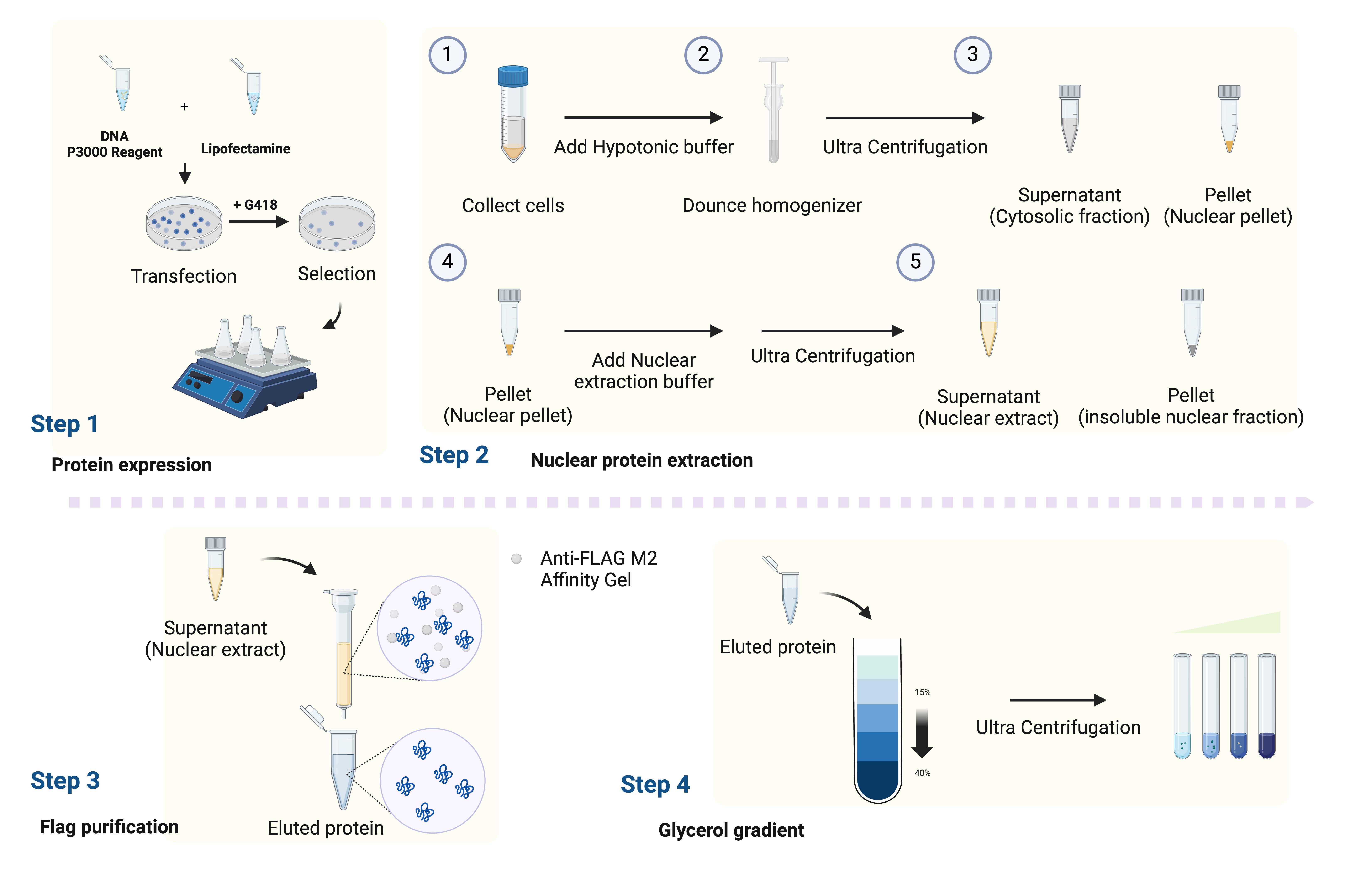
Workflow for isolating the endogenous Mediator complex. The workflow outlines the steps from generating the stable cell line to protein purification.
Background
In eukaryotes, the Mediator complex is an evolutionarily conserved transcriptional coactivator composed of 25 subunits in budding yeast and 30 subunits in humans [1]. The Mediator complex consists of a large core (cMED) with three domains: the head, middle, and tail, along with a dissociable module known as the CDK8 kinase module (CKM). The CKM contains four subunits: cyclin-dependent kinase 8 (CDK8), Cyclin C, MED12, and MED13 [2]. The primary function of cMED is to activate Pol II–dependent transcription by recruiting Pol II to promoters [3]. However, this activity is repressed by the CKM through steric hindrance mediated by MED13 [4,5]. Interestingly, recent studies suggest that CDK8 also plays a positive role in gene transcription, though the mechanisms remain poorly understood. Since the CKM serves as a key entry point for developmental and oncogenic signaling via the Mediator complex [6], isolating the CKM-cMED complex is essential for investigating its mechanisms and functions, providing deeper insights into these processes.
The human CKM-cMED complex consists of 30 subunits, and overexpressing each individually requires considerable effort. As a transcriptional coactivator, the Mediator interacts with multiple binding partners, such as RNA pol II, further complicating the process of isolating pure CKM-cMED complex. Therefore, we developed a protocol to obtain a high-quality and large quantity of CKM-cMED complex.
Previously, we established a stable HEK293 cell line expressing CDK8. However, scaling up proved challenging due to the limitations associated with adherent cells. Then, we selected the FreeStyle 293-F cells suspension cell culture system for its ease of expansion and high protein expression yields. Since CKM and Pol II are mutually exclusive for cMED binding, we overexpressed the CDK8, one of the CKM subunits, with a C-terminal FLAG tag, but not any subunits of cMED, in freestyle 293-F cells to prevent RNA Pol II contamination. The size of the FLAG tag, consisting of eight amino acids, is small and specifically recognized by the antibody conjugated to agarose beads. Additionally, the FLAG tag added to the C-terminus of CDK8 did not compromise the stability of the CKM-cMED complex and still maintained its kinase activity. By establishing stable cell lines through antibiotic selection, we could stably express FLAG-tagged recombinant CDK8 in FreeStyle 293-F cells, enabling the collection of large quantities of cells for protein purification.
For protein purification, we began by isolating nuclear extract to enrich the CKM-cMED complex as it is primarily present in the nucleus. Affinity purification was then performed using anti-FLAG M2 affinity gel to ensure sufficient purity and quality to proceed to the next step. To enhance the homogeneity of the protein complex, we further performed glycerol gradient centrifugation without a cross-linker and successfully obtained the functional endogenous CKM-cMED complex. Because the molecular weight of CKM-cMED is very large, density gradient sedimentation is an effective method to separate the complex from relatively small degradation products, disassembled components, and other impurity proteins present in the FLAG elution. This protocol provides a reliable method for isolating the homogenous endogenous CKM-cMED complex from FLAG-CDK8 expressed cells using FLAG-specific immunoprecipitation and glycerol gradient sedimentation for future investigations of structure-function relationships.
Materials and reagents
Biological materials
1. FreestyleTM 293-F cells (Thermo Fisher, catalog number: R79007), containing 1 × 10 cells suspended in 90% FreeStyleTM 293 expression medium and 10% DMSO, stored in liquid nitrogen
2. Plasmid DNA (pcDNA3.1_CDK8-F)
Reagents
1. Lipofectamine 3000 (Invitrogen, catalog number: L3000001)
2. G418 sulfate (Thermo Fisher, catalog number: 11811023)
3. ANTI-FLAG® M2 affinity gel (Sigma, catalog number: A2220)
4. cOmpleteTM, mini, EDTA-free protease inhibitor cocktail (Sigma, catalog number: 50-161-3323)
5. FreeStyleTM 293 expression medium (prewarm to 37 °C before use)
6. Phosphate buffered saline (PBS), 1×, liquid, pH 7.4 (GenDEPOT, catalog number: P2201-050)
7. HEPES (Merck, catalog number: 391338)
8. Magnesium chloride (Sigma, catalog number: M8266)
9. Potassium chloride (Sigma, catalog number: P3911)
10. Dithiothreitol (DTT) (Fisher Scientific, catalog number: BP172-5)
11. Protease inhibitor cocktail (RPI, catalog number: P50600-1)
12. Ethylenediaminetetraacetic acid (EDTA) (Sigma, catalog number: E6758)
13. NP-40 Surfact-AmpsTM detergent solution (Thermo Fisher, catalog number: 28324)
14. Glycerol (Fisher Scientific, catalog number: BP229-4)
15. Sodium chloride (Sigma, catalog number: 567440)
16. beta-mercaptoethanol (Sigma, catalog number: 444203)
17. DYKDDDDK tag Peptide (FLAG peptide) (APExBio, catalog number: A6002)
Solutions
1. Hypotonic buffer (see Recipes)
2. Nuclear extraction buffer (see Recipes)
3. Washing buffer A (see Recipes)
4. Washing buffer B (see Recipes)
5. Washing buffer C (see Recipes)
6. Elution buffer (see Recipes)
7. 15% glycerol gradient buffer (see Recipes)
8. 40% glycerol gradient buffer (see Recipes)
Recipes
1. Hypotonic buffer
10 mM HEPES pH 7.9
1.5 mM MgCl2
10 mM KCl
0.5 mM dithiothreitol (DTT)
Protease inhibitors (freshly added)
2. Nuclear extraction buffer
25 mM HEPES pH 7.9
1.5mM MgCl2
0.6 M KCl
0.5 mM DTT
0.2 mM EDTA
0.02% NP-40
20% glycerol
Protease inhibitors (freshly added)
3. Washing buffer A
25 mM HEPES pH 7.6
1.5 mM MgCl2
150 mM NaCl
10% glycerol
0.2 mM EDTA
2 mM beta-mercaptoethanol
Protease inhibitors (freshly added)
4. Washing buffer B
25 mM HEPES pH 7.6
1.5 mM MgCl2
150 mM NaCl
10% glycerol
0.2 mM EDTA
2 mM beta-mercaptoethanol
5. Washing buffer C
25 mM HEPES pH 7.6
1.5 mM MgCl2
150 mM NaCl
10% glycerol
0.2 mM EDTA
0.01% NP40
2 mM beta-mercaptoethanol
6. Elution buffer
25 mM HEPES pH 7.6
1.5 mM MgCl2
150 mM NaCl
10% glycerol
0.2 mM EDTA
0.01% NP40
2 mM beta-mercaptoethanol
200 μg/mL 1× FLAG peptide
7. 15% glycerol gradient buffer
20 mM HEPES pH 7.6
1 mM MgCl2
300 mM NaCl
0.25 mM EDTA
1 mM beta-mercaptoethanol
15% (v/v) glycerol
8. 40% glycerol gradient buffer
20 mM HEPES pH 7.6
1 mM MgCl2
300 mM NaCl
0.25 mM EDTA
1 mM beta-mercaptoethanol
40% (v/v) glycerol
Filter sterilize and store all buffers at 4 °C.
Laboratory supplies
1. Clean, sterile glass 250, 1,000, 2,000 mL flasks
2. Hemacytometer
3. 60 × 15 mm non-treated culture dish, sterile (CytoOne, catalog number: CC7672-3359)
4. 15 and 50 mL conical sterile polypropylene centrifuge tubes (Thermo Scientific, catalog number: 12-565-269, 12-565-271)
5. Dounce homogenizer
6. 1.7 mL microtubes (e.g., Axygen, catalog number: 14-222-168)
7. Centrifuge bottles with sealing closure (Thermo Scientific, catalog number: 05-564-3)
8. Polycarbonate ultracentrifuge bottles (Beckman, catalog number: 355622)
9. 4 mL ultra-clear polypropylene tubes (Beckman, catalog number: 344062)
10. Econo-Pac® chromatography columns (Bio-Rad, catalog number: 7321010)
11. NovexTM tris-glycine mini protein gels, 4%–20%, 1.0 mm, WedgeWellTM format (Invitrogen, catalog number: XP04205BOX)
Equipment
1. Standard orbital shaker (VWR, model: 1000, catalog number: 89032-088)
2. CO2 cell culture incubator
3. Inverted microscope
4. Biological safety cell culture hood
5. 37 °C water bath
6. Benchtop centrifuge (Eppendorf, catalog number: 022625501)
7. High-speed centrifuge (Thermo Sorvall, model: RC-6 Plus; PTI FiberLite F10-6x500y fixed angle rotor)
8. Ultracentrifuges (Beckman, model: Optima XPN-90; 45 Ti fixed-angle titanium rotor, SW60 Ti swinging-bucket rotor)
9. GRADIENT MASTERTM (Biocomp, Model 108)
10. Tube revolver rotator (Thermo Scientific, catalog number: 88881001)
Software and datasets
1. BioRender (https://www.biorender.com/) was used for the Graphical overview: Tang, H. (2025) https://BioRender.com/z21d813; and Figure 3: Tang, H. (2025) https://BioRender.com/t82q193
Procedure
文章信息
稿件历史记录
提交日期: Oct 3, 2024
接收日期: Dec 3, 2024
在线发布日期: Jan 1, 2025
出版日期: Feb 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Tang, H. C., Tsai, K. L. and Chao, T. C. (2025). A Protocol to Purify Human Mediator Complex From Freestyle 293-F Cells. Bio-protocol 15(4): e5185. DOI: 10.21769/BioProtoc.5185.
分类
生物化学 > 蛋白质 > 分离和纯化
生物化学 > 蛋白质 > 结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link