- EN - English
- CN - 中文
Isolation of Intact Mitochondria From Drosophila melanogaster and Assessment of Mitochondrial Respiratory Capacity Using Seahorse Analyzer
果蝇完整线粒体的分离及其线粒体呼吸能力的Seahorse分析
发布: 2025年02月05日第15卷第3期 DOI: 10.21769/BioProtoc.5180 浏览次数: 2241
评审: Darrell CockburnAnnmary Paul ErinjeriAnonymous reviewer(s)

相关实验方案

利用Seahorse XFe96通量分析仪检测PBMC的线粒体应激反应,并比较Poly-D-Lysine与Poly-L-Lysine的细胞附着性能
Kumudu Subasinghe [...] Nicole Phillips
2025年06月05日 1970 阅读
Abstract
Analysis of mitochondrial function has broad applicability in many research specialties. Neurodegenerative disorders such as chemotherapy-induced peripheral neuropathy (CIPN) often exhibit damaged mitochondria or reduced mitochondrial respiratory capacity. Isolation of intact mitochondria for protein analysis or respiration measurements has been previously reported in numerous model organisms. Here, we describe an adaptation of previous protocols to isolate intact functional mitochondria from Drosophila melanogaster for use in a model of CIPN. Whole Drosophila are ground in isolation buffer, and mitochondria are purified using differential centrifugation through a sucrose and mannitol solution. The intact mitochondria are plated as a monolayer for measurements of mitochondrial oxygen consumption rates and response to inhibitor compounds on an Agilent Seahorse analyzer. This experimental protocol is quick and yields a purified population of intact mitochondria that may be used for functional assays for several hours after isolation. The isolated mitochondria may be used for respiration measurements, which reflect their health, and stored for protein or genetic analysis. Mitochondrial populations from multiple strains or treatment groups can be easily compared simultaneously. The rapid biochemical assessment of mitochondria, in combination with the utility of Drosophila as an in vivo genetic model system, offers great potential for researchers to probe the impact of genetics and pharmacologic interventions on mitochondrial respiratory capacity.
Key features
• This protocol describes rapid isolation of intact, functional mitochondria that may be used for respiration measurements or other biochemical analyses.
• Mitochondria isolated from Drosophila are assessed in an Agilent Seahorse analyzer utilizing multiple substrates and electron transport chain inhibitors to fully characterize mitochondrial respiratory capacity.
• This protocol is optimized to use Drosophila for easy in vivo genetic and pharmacologic manipulation, and assessment of the impact on mitochondrial function.
Keywords: Mitochondria (线粒体)Graphical overview
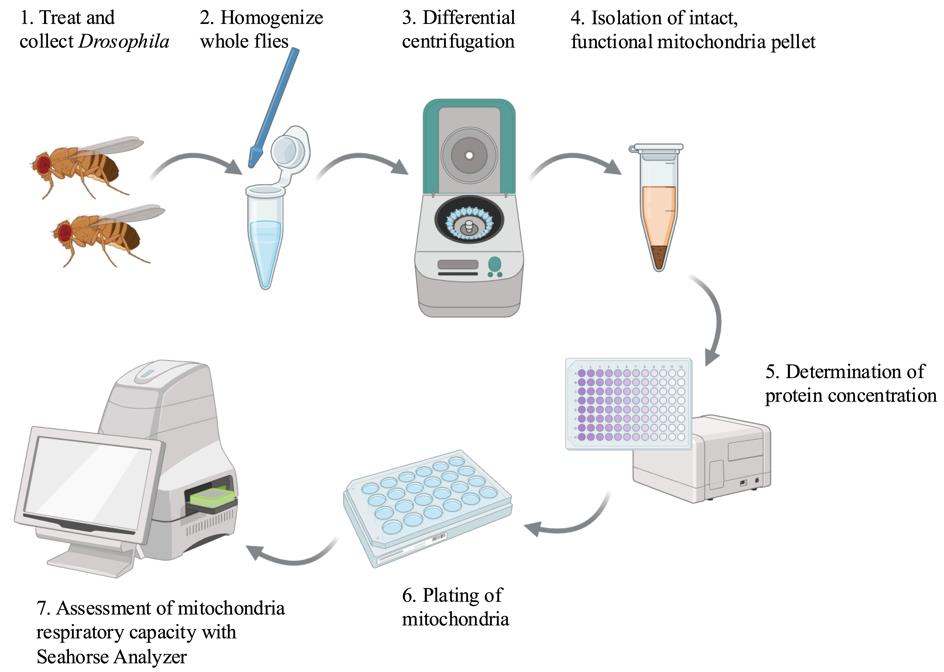
Created in BioRender.com
Background
Mitochondria function is increasingly recognized as an essential factor in human health and disease. Altered mitochondria function is observed in cancer [1], aging [2–4], metabolic disorders [5], and neurodegenerative disorders such as Parkinson’s Disease [6] and chemotherapy-induced neuropathy [7,8]. Assessment of mitochondrial function and cellular respiration rates can reveal disease states in an organism or tissue. These measurements are invaluable tools to parse the reactions of cells to acute toxic stressors, genetic manipulations, or potential preventive and therapeutic interventions [9]. Analysis of mitochondrial health has progressed from earlier techniques using Clark electrodes to measure O2 consumption in individual samples to more advanced instrumentation such as Agilent Seahorse analyzers, which are capable of simultaneous analysis of 96-well plates. Modern respiration analyzers can also measure real-time response to the addition of substrates, metabolites, and mitochondria inhibitors. Mitochondria respiration analysis has been measured using intact cells in cell culture plates, dissociated tissues, and isolated mitochondria. Insights gained from these experiments advance our understanding of the role of mitochondria in disease and provide new potential biomarkers for the assessment of disease states [9].
Previous publications have described detailed protocols for the measurement of cellular respiration [9–11], isolation of mitochondria for metabolic analysis [12], and use of isolated mitochondria from mouse muscle tissue for oxygen consumption rate analysis in Seahorse analyzers [13]. Drosophila-specific protocols in this field include detailed experimental methods for isolation of larval mitochondria [14], metabolic analysis of flight muscle mitochondria [12], and isolation of mitochondria for enzymatic analysis of electron transport chain components [15]. The protocol described here is a modification of previous mitochondria isolation procedures from Drosophila, with optimization of Seahorse analysis of these isolated mitochondria to assess differences in respiratory capacity between different fly strains treated with chemotherapy agents known to damage mitochondria. This protocol was previously described in a manuscript reporting differences in cisplatin sensitivity between Drosophila strains and showed that the mitochondria have different basal respiration rates and different abilities to maintain activity following treatment with toxic stressors like cisplatin [16].
The protocol described here allows for rapid isolation of a pure mitochondria pellet. The mitochondria retain their activity for several hours after isolation if kept on ice. In addition, the intact mitochondria may be used for protein analysis or enzymatic activity assays. Isolation of mitochondria allows for direct assessment of electron transport chain function and the impact of multiple substrates and inhibitors. This allows researchers to focus on specific aspects of cell metabolism without the variables presented by whole-cell metabolomics. The availability of substrates can be tightly controlled by the user, whereas whole-cell or tissue respiration measurements must consider the availability of metabolites contributed by the whole-cell environment. ADP is often used as an injection compound to measure mitochondrial respiration before and after the substrate for ATP synthase is available. Respiration through Complex I of the electron transport chain may be measured through the use of pyruvate and malate as initial substrates. Succinate can be used as a substrate for Complex II, and ascorbate is a substrate for Complex IV. Likewise, inhibitors block components of the electron transport chain. Rotenone and antimycin A block Complex I and Complex III, respectively. Oligomycin blocks the activity of ATP synthase, while FCCP uncouples oxygen consumption from ATP synthesis. Careful selection of substrates and inhibitors can reveal a great deal about the function of isolated mitochondria. This method does not, however, provide a complete picture of cellular respiration, as the mitochondria are removed from the context of the cell. The lack of whole cells limits data collection from the Seahorse to oxygen consumption rate (OCR). The extracellular acidification rate (ECAR) measured by the Seahorse as a readout of glycolytic activity is not useful when only assessing isolated mitochondria. Researchers should carefully choose an experimental protocol based on specific goals in characterizing cellular metabolism.
Materials and reagents
Biological materials
1. Drosophila melanogaster stocks (Bloomington Drosophila Stock Center, stock numbers 36303 and 36304 were used in the sample data)
Reagents
1. Sucrose (Sigma-Aldrich, catalog number: S1888-1KG)
2. D-Mannitol (Sigma-Aldrich, catalog number: M4125-100G)
3. HEPES buffer, 1 M, pH 7.2 (Corning, catalog number: 25-060-Cl)
4. EDTA, 0.5 M (Sigma-Aldrich, catalog number: 03690-100ML)
5. Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: 05470-5G)
6. Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: 221473-25G)
7. Monobasic potassium phosphate (KH2PO4) (Sigma-Aldrich, catalog number: 1551139-5G)
8. Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266-100G)
9. EGTA, 0.5 M (RPI, catalog number: E14100-50.0)
10. Pyruvic acid (Sigma-Aldrich, catalog number: 107360-25G)
11. Malic acid (Sigma-Aldrich, catalog number: M6413-25G)
12. Adenosine 5’-diphosphate monopotassium salt dihydrate (ADP) (Sigma-Aldrich, catalog number: A5285)
13. Oligomycin A (Sigma-Aldrich, catalog number: 75351)
14. Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) (Sigma-Aldrich, catalog number: C2920)
15. Rotenone (Sigma-Aldrich, catalog number: R8875)
16. Antimycin A (Sigma-Aldrich, catalog number: A8674)
17. Seahorse XF calibrant (Agilent, catalog number: 100840-000)
18. DC Protein Assay Kit I (Bio-Rad, catalog number: 5000111)
19. 95% Ethanol (Millipore-Sigma, catalog number: 65348-M)
Solutions
1. 10% w/v BSA (see Recipes)
2. Pyruvate stock solution (see Recipes)
3. Malate stock solution (see Recipes)
4. ADP stock solution (see Recipes)
5. Oligomycin A stock solution (see Recipes)
6. FCCP stock solution (see Recipes)
7. Rotenone stock solution (see Recipes)
8. Antimycin A stock solution (see Recipes)
9. Mitochondria isolation buffer (see Recipes)
10. Mitochondria assay buffer (2× stock) (see Recipes)
11. Working mitochondria assay buffer (1×) with substrates (see Recipes)
Recipes
1. 10% w/v BSA
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| BSA | 10% w/v | 1 g |
| ddH2O | n/a | * |
| Total (optional) | n/a | 10 mL |
*Fully dissolve BSA in ~5–7 mL of ddH2O and then bring the solution to a final volume of 10 mL.
Aliquot and store at -20 °C.
2. Pyruvate stock solution (100 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Pyruvic acid | 100 mM | 0.8806 g |
| ddH2O | n/a | 100 mL |
| Total (optional) | n/a | 100 mL |
Aliquot and store at -20 °C.
3. Malate stock solution (100 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Malic acid | 100 mM | 1.34 g |
| 95% ethanol | n/a | 100 mL |
| Total (optional) | n/a | 100 mL |
Aliquot and store at -20 °C.
4. ADP stock solution (100 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| ADP | 100 mM | 5.0132 g |
| ddH2O | n/a | 100 mL |
| Total (optional) | n/a | 100 mL |
Aliquot and store at -20 °C.
5. Oligomycin A stock solution (10 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Oligomycin A | 10 mM | 0.079 g |
| 95% ethanol | n/a | 10 mL |
| Total (optional) | n/a | 10 mL |
Aliquot and store at -20 °C.
6. FCCP stock solution (10 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| FCCP | 10 mM | 0.025 g |
| 95% ethanol | n/a | 10 mL |
| Total (optional) | n/a | 10 mL |
Aliquot and store at -20 °C.
7. Rotenone stock solution (5 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Rotenone | 5 mM | 0.019 g |
| 95% ethanol | n/a | 10 mL |
| Total (optional) | n/a | 10 mL |
Aliquot and store at -20 °C.
8. Antimycin A stock solution (10 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Antimycin A | 10 mM | 0.055 g |
| 95% ethanol | n/a | 10 mL |
| Total (optional) | n/a | 10 mL |
Aliquot and store at -20 °C.
9. Mitochondria isolation buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sucrose | 70 mM | 4.79 g |
| D-Mannitol | 210 mM | 7.65 g |
| 1 M HEPES, pH 7.2 | 5 mM | 1 mL |
| 0.5 M EDTA | 1 mM | 0.4 mL |
| 10% BSA | 0.5% | 10 mL* |
| KOH | n/a | pH to 7.2 |
| ddH2O | n/a | ** |
| Total (optional) | n/a | 200 mL |
*BSA is included in the isolation buffer and assay buffers to bind free fatty acids released during tissue homogenization and helps to maintain mitochondrial membrane potential and respiratory function.
**Fully dissolve solid reagents in ~100 mL of ddH2O. Use KOH to bring the pH to 7.2 and then bring the solution to a final volume of 200 mL.
Aliquot and store at -20 °C
10. Mitochondria assay buffer (2× stock)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sucrose | 140 mM | 9.58 g |
| D-Mannitol | 440 mM | 15.3 g |
| KH2PO4 | 20 mM | 0.544 g |
| MgCl2 | 10 mM | 0.19 g |
| 1 M HEPES, pH 7.2 | 4 mM | 0.80 mL |
| 0.5 M EGTA | 2 mM | 0.80 mL |
| 10% BSA | 0.4% | 8 mL |
| KOH | n/a | pH to 7.2 |
| ddH2O | n/a | * |
| Total (optional) | n/a | 200 mL |
*Fully dissolve solid reagents in ~100 mL of ddH2O. Use KOH to bring the pH to 7.2 and then bring the solution to a final volume of 200 mL.
Aliquot and store at -20 °C
11. Working mitochondria assay buffer (1×) with pyruvate and malate
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Mitochondria assay buffer (2× stock) | 1× | 15 mL |
| 100 mM pyruvate stock solution | 10 mM | 3 mL |
| 100 mM malate stock solution | 5 mM | 1.5 mL |
| ddH2O | n/a | 10.5 mL |
| Total (optional) | n/a | 30 mL |
Stock solutions and buffers should be aliquoted and stored at -20 °. Make this solution fresh on the day of the assay.
Laboratory supplies
1. 1.5 mL Eppendorf tubes (Sigma-Aldrich, catalog number: EP022363212)
2. 15 mL centrifuge tubes (Corning, catalog number: CLS430052)
3. 50 mL centrifuge tubes (Corning, catalog number: CLS430828)
4. Pestles (Sigma-Aldrich, catalog number: BAF199230000)
5. Seahorse FluxPak (Agilent)
Note: This protocol describes experiments performed with Seahorse XF24 FluxPaks. This model has been discontinued and replaced by the Seahorse XFe24 analyzer and FluxPaks (Agilent, catalog number: 102340-100)
6. 96-well clear flat bottom microplates (Corning, catalog number: 353072)
Equipment
1. Sorvall Legend X1R centrifuge (Thermo Scientific, catalog number: 75004220)
2. M-20 microplate swinging bucket rotor (Thermo Scientific, catalog number: 75003624)
3. Eppendorf Centrifuge 5425 (Sigma-Aldrich, catalog number: 5405000646)
4. SpectraMax M3 microplate reader (Molecular Devices, catalog number: M3)
5. CO2 tank (for Drosophila anesthesia)
6. CO2 regulator (for Drosophila anesthesia) (Flystuff, catalog number: 59-143)
7. CO2 fly pad (Flystuff, catalog number: 59-114)
8. CO2 blowgun (Flystuff, catalog number: 54-104)
9. Fly brushes (Flystuff, catalog number: 59-204)
10. Fisher Scientific Isotemp incubator, non-CO2 (Fisher Scientific, catalog number: 15-103-0514)
11. Seahorse XF24 analyzer (Agilent)
Note: The current equivalent model Agilent Seahorse is the XFe24. Any Agilent Seahorse will be able to measure oxygen consumption from isolated mitochondria. Volumes and concentrations of reagents should be optimized for the analyzer.
Software and datasets
1. WAVE Desktop v2.6 (Agilent, 2018)
2. Prism v10.3.1 (GraphPad, 2024)
3. Microsoft Excel (Office 365, 2024)
Procedure
文章信息
稿件历史记录
提交日期: Oct 5, 2024
接收日期: Nov 28, 2024
在线发布日期: Dec 20, 2024
出版日期: Feb 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Groen, C. M. and Windebank, A. J. (2025). Isolation of Intact Mitochondria From Drosophila melanogaster and Assessment of Mitochondrial Respiratory Capacity Using Seahorse Analyzer. Bio-protocol 15(3): e5180. DOI: 10.21769/BioProtoc.5180.
- Groen, C. M., Podratz, J. L., Pathoulas, J., Staff, N. and Windebank, A. J. (2021). Genetic Reduction of Mitochondria Complex I Subunits is Protective against Cisplatin-Induced Neurotoxicity in Drosophila. J Neurosci. 42(5): 922–937. https://doi.org/10.1523/jneurosci.1479-20.2021
分类
神经科学 > 细胞机理 > 线粒体
细胞生物学 > 细胞器分离 > 线粒体
细胞生物学 > 细胞新陈代谢 > 呼吸测量法
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link