- EN - English
- CN - 中文
Detection of Amylin-β-amyloid Hetero-Oligomers by Enzyme-Linked Immunosorbent Assay
酶联免疫吸附法检测胰淀素-β淀粉样蛋白异源寡聚体
发布: 2025年02月05日第15卷第3期 DOI: 10.21769/BioProtoc.5179 浏览次数: 1415
评审: Olga KopachRupkatha BanerjeeAmberley D. StephensAnonymous reviewer(s)

相关实验方案
Cluster FLISA——用于比较不同细胞系蛋白表达效率及蛋白亚基聚集状态的方法
Sabrina Brockmöller and Lara Maria Molitor
2025年11月05日 1203 阅读
Abstract
Amylin is an amyloidogenic neuroendocrine hormone co-synthesized and co-secreted with insulin from the pancreas. It readily crosses the blood–brain barrier and synergistically forms mixed amyloid plaques with β-amyloid (Aβ) in brain parenchyma. Parenchymal amylin-Aβ plaques are found in both sporadic and early-onset familial Alzheimer’s disease (AD), yet their (patho)physiological role remains elusive, particularly due to a lack of detection modalities for these mixed plaques. Previously, we developed an enzyme-linked immunosorbent assay (ELISA) capable of detecting amylin-Aβ hetero-oligomers in brain lysate and blood using a polyclonal anti-amylin antibody to capture hetero-oligomers and a monoclonal anti-Aβ mid-domain detection antibody combination. This combination allows for the recognition of distinct amylin epitopes, which remain accessible after amylin-Aβ oligomerization has begun, and precise detection of Aβ epitopes available after oligomer formation. The utility of this assay is evidenced in our previous report, wherein differences in hetero-oligomer content in brain tissue from patients with and without AD and patients with and without diabetes were distinguished. Additionally, using AD model rats, we provided evidence that our assay can be employed for the detection of amylin-Aβ in blood. This assay and protocol are important innovations in the field of AD research because they meet an unmet need to detect mixed amyloid plaques that, if targeted therapeutically, could reduce AD progression and severity.
Key features
• Detects amylin-Aβ hetero-oligomers in blood from patients with Alzheimer’s disease.
• Enables simultaneous, high-throughput analysis of hetero-oligomer content of brain and blood tissue.
• Allows exploration into the amylin-Aβ interaction during AD pathogenesis, potentially leading to novel treatment mechanisms by controlling the amylin-Aβ interaction.
Keywords: Amylin (胰淀素)Graphical overview
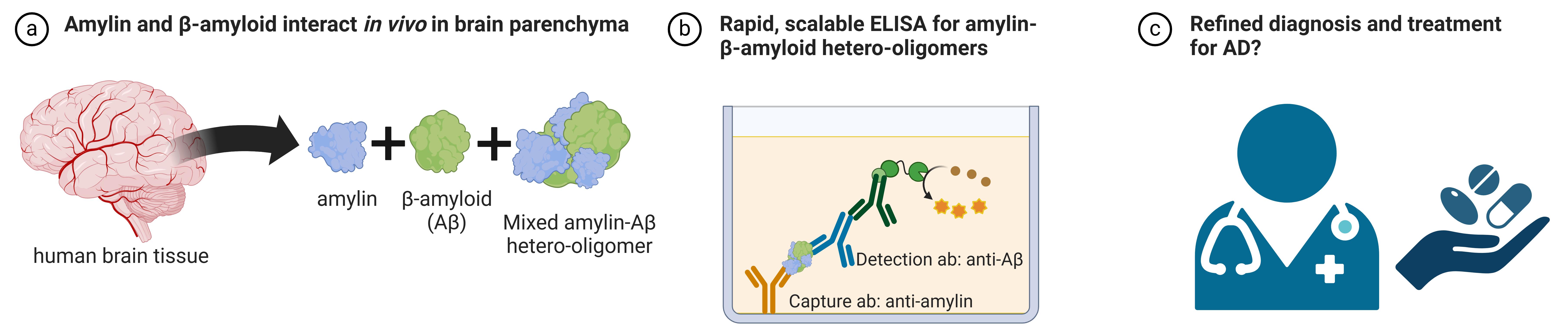
Detection of amylin-Aβ hetero-oligomers by ELISA. (A) Amyloidogenic amylin and Aβ form mixed hetero-oligomers in brain tissue. (B) The ELISA described in this protocol is capable of detecting these mixed hetero-oligomers. (C) In the future, perhaps the detection of these mixed hetero-oligomers can assist in refining diagnostic measures and treatments for Alzheimer’s disease.
Background
Alzheimer's disease (AD) has previously been linked to dysregulated pancreatic hormones, including insulin, which can exacerbate AD pathology by causing brain insulin resistance [1–2]. Amylin, a centrally acting neuroendocrine hormone co-synthesized and co-secreted with insulin from pancreatic β-cells [3], readily crosses from the blood to the brain parenchyma [4] and is reportedly involved in the central regulation of satiation [5]. In nearly all (>95% prevalence) patients with type-2 diabetes mellitus (T2DM), amylin forms cytotoxic amyloid plaques in the pancreas, which contributes to the pathogenesis of T2DM by inducing β-cell apoptosis and subsequent β-cell mass depletion [6–8]. In both sporadic and early-onset familial AD, amylin synergistically forms co-aggregates with vascular and brain parenchymal Aβ in the brain [9–14]. Consistent with findings in human AD brains, APPswe/PS1dE9 (APP/PS1) rats expressing human amylin in the pancreas (since murine amylin is non-amyloidogenic) revealed that chronic exposure to circulating amylin promoted cerebrovascular and parenchymal amylin-Aβ deposition [9–13]. These results suggest an association between Aβ pathology and amyloidogenic human amylin but the exact mechanism responsible for amylin-Aβ co-aggregation remains to be determined.
Previous research demonstrated that heterologous seeding between amylin and common Aβ fragments (e.g., Aβ42 and Aβ40) promotes amyloid formation in a manner that is comparable to that of homogenous amylin amyloid [15–17]. In vivo amylin-Aβ hetero-amyloid formation and cytotoxicity are facilitated by the co-expression of the two peptides in cells [21–22]. The hypothesis that amylin and Aβ peptides aggregate in the human AD brain to form amylin-Aβ hetero-oligomers has previously been verified by co-immunoprecipitation experiments using human brain samples from AD patients [9]. These converging data [9,15–22] support the presence of amylin-Aβ hetero-oligomers in the human brain and establish a need for their quantification. Proper detection and quantification of amylin-Aβ hetero-oligomers may further establish the connection between amylin and Aβ and their collective role in AD pathology.
While the need for a means to reliably quantify the molecular amylin-Aβ interaction in AD has been acknowledged [9–13], currently employed molecular techniques (namely immunoprecipitation, western blot, circular dichroism, and electron microscopy) limit studies to reduced sample sizes due to their inherent complexity, non-scalability, and in some cases, cost. Additionally, the denaturing conditions commonly used in methods such as western blot prove to be additional obstacles, as the size and structural stability of hetero-oligomers can be altered. Therefore, there is still an unmet need for an assay method that can detect and quantify hetero-oligomers of amylin-Aβ without interrupting molecular interactions. Previously, we reported the creation of a novel enzyme-linked immunosorbent assay (ELISA) to detect amylin-Aβ hetero-oligomers in blood and brain tissue [23] in AD model animals and human donor tissue. Here, we provide a detailed, stepwise protocol for our assay. Relative to the currently employed techniques, this assay is scalable, high-throughput, cost-effective, and non-complex, all while critically maintaining hetero-oligomer stability. The assay detailed here may prove to be a useful tool to detect, analyze, and eventually disentangle the role of the amyloidogenic amylin and Aβ proteins in AD and other pathological conditions.
Materials and reagents
Biological materials
1. Mouse anti-human-Aβ(1-16) antibody (BioLegend, catalog number: 803002)
2. Rabbit anti-amylin P2 antibody (not commercially available, see [23])
3. Mouse anti-human-total-Aβ (BioLegend, catalog number: 800720)
4. Aβ40 synthetic peptide (Anaspec, catalog number: AS-24235)
Reagents
1. ELISA assay diluent (5×) (BioLegend, catalog number: 421203)
2. Dimethyl sulfoxide (DMSO) (Sigma, catalog number: D8418)
3. Na2CO3 (Sigma, catalog number: S7795)
4. NaHCO3 (Sigma, catalog number: S5761)
5. 10× PBS (Corning, catalog number: 46-013-CM)
6. 10% Tween-20 (Bio-Rad, catalog number: 1610781)
7. Aβ40 synthetic peptide (Anaspec, catalog number: AS-24235)
8. 3,3',5,5' tetramethylbenzidine (TMB) substrate (Thermo Scientific, catalog number: 34028)
9. Stop solution (Thermo Scientific, catalog number: N600)
Solutions
1. Bicarbonate buffer (see Recipes)
2. PBST washing buffer (see Recipes)
Recipes
1. Bicarbonate buffer (250 mL)
Note: Adjust the solution’s pH to 9.6. Use distilled water as diluent.
| Reagent | Final concentration | Mass |
|---|---|---|
| Na2CO3 | 0.028 M | 0.7575 g |
| NaHCO3 | 0.071 M | 1.50 g |
2. PBST washing buffer (500 mL)
Note: Dilute 50 mL of 10× PBS to 1× using 450 mL of distilled water as diluent before preparing PBST washing buffer.
| Reagent | Final concentration | Volume |
|---|---|---|
| 1× PBS | - | 500 mL |
| 10% Tween-20 | 0.05% | 2.5 mL |
Laboratory supplies
1. 96-well clear flat-bottom polystyrene high bind microplate (Corning, catalog number: 9018)
2. Sealing film, polyester (VWR, catalog number: 89134-430)
3. Absorbent paper towels (Pacific Blue Basic, catalog number: 23504)
4. epT.I.P.S. standard 20–300 μL pipette tips (Eppendorf, catalog number: 022492047)
5. 1.5 mL standard line microcentrifuge tubes (VWR, catalog number:10025-726)
6. 50 mL reagent reservoirs (Corning, catalog number: 4870)
7. 8-channel 30–300 μL multichannel pipette (USA Scientific, catalog number: 7108-3300)
Equipment
1. xMark microplate absorbance spectrophotometer (Bio-Rad, catalog number: 1681150)
Software and datasets
1. Microsoft Excel (2019)
2. Microplate Manager Software 6 (MPM) (Bio-Rad, v6.3)
Procedure
文章信息
稿件历史记录
提交日期: Aug 8, 2024
接收日期: Dec 5, 2024
在线发布日期: Dec 26, 2024
出版日期: Feb 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Leibold, N. S., Kotiya, D., Verma, N. and Despa, F. (2025). Detection of Amylin-β-amyloid Hetero-Oligomers by Enzyme-Linked Immunosorbent Assay. Bio-protocol 15(3): e5179. DOI: 10.21769/BioProtoc.5179.
- Kotiya, D., Leibold, N., Verma, N., Jicha, G. A., Goldstein, L. B. and Despa, F. (2023). Rapid, scalable assay of amylin-β amyloid co-aggregation in brain tissue and blood. J Biol Chem. 299(5): 104682.
分类
神经科学 > 神经系统疾病 > 神经退行性病变
神经科学 > 基础技术 > 高通量筛选
生物化学 > 蛋白质 > 免疫检测
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link