- EN - English
- CN - 中文
Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice
小鼠耳蜗器官解剖、免疫染色及共聚焦成像
(*contributed equally to this work) 发布: 2025年01月20日第15卷第2期 DOI: 10.21769/BioProtoc.5167 浏览次数: 3747
评审: Marion HoggMohammed Mostafizur RahmanAnonymous reviewer(s)
Abstract
The organ of Corti, located in the inner ear, is the primary organ responsible for animal hearing. Each hair cell has a V-shaped or U-shaped hair bundle composed of actin-filled stereocilia and a kinocilium supported by true transport microtubules. Damage to these structures due to noise exposure, drug toxicity, aging, or environmental factors can lead to hearing loss and other disorders. The challenge when examining auditory organs is their location within the bony labyrinth and their small and fragile nature. This protocol describes the dissection procedure for the cochlear organ, followed by confocal imaging of immunostained endogenous and fluorescent proteins. This approach can be used to understand hair cell physiology and the molecular mechanisms required for normal hearing.
Key features
• Protocol for the microdissection of the organ of Corti and suitable preparation for later immunostaining.
• This technique involves the evaluation of mouse cochlea for planar-cell-polarity protein.
• Quantitative and qualitative analysis of hair cell cilia in different dimensions.
Keywords: Cochlea (耳蜗)Graphical overview
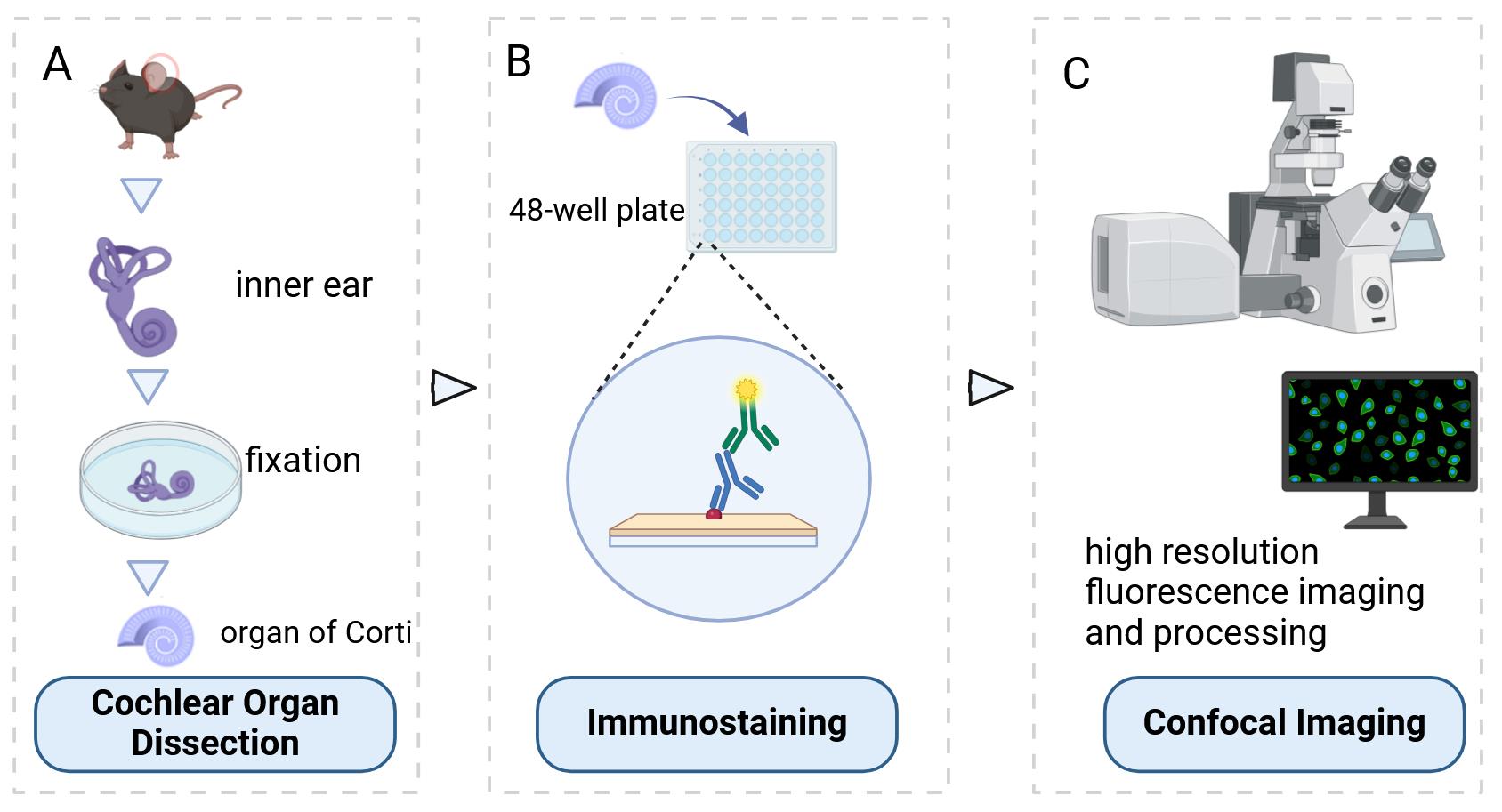
Background
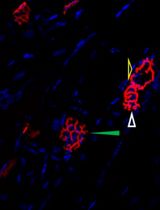
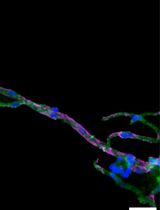
The inner ear, serving as both the auditory organ and local sensory receptor, houses the critical auditory sensor [1,2]. It consists of three rows of outer hair cells and one row of inner hair cells, as well as various supporting cells. These cellular components are meticulously arrayed in a mosaic pattern on the intricate cochlear membrane of the inner ear, demonstrating remarkable flexibility [3–6]. Hair cells convert acoustic signals into electrical signals through the deflection of tip cilia. These cilia are important cellular structures involved in normal morphogenesis and functional activities of hair cells [7,8]. Electrical impulses are ultimately channeled to the cerebral cortex, facilitating auditory perception. Research shows that the primary cause of hearing loss is the involuntary regeneration of damaged hair cells in the mammalian inner ear [9]. The investigation into the auditory impairment mechanism hinges upon the intricate functioning of the Corti organ. Advances in employing specialized immunochemical techniques have significantly enhanced our comprehension of its fundamental operating principles (Figure 1).
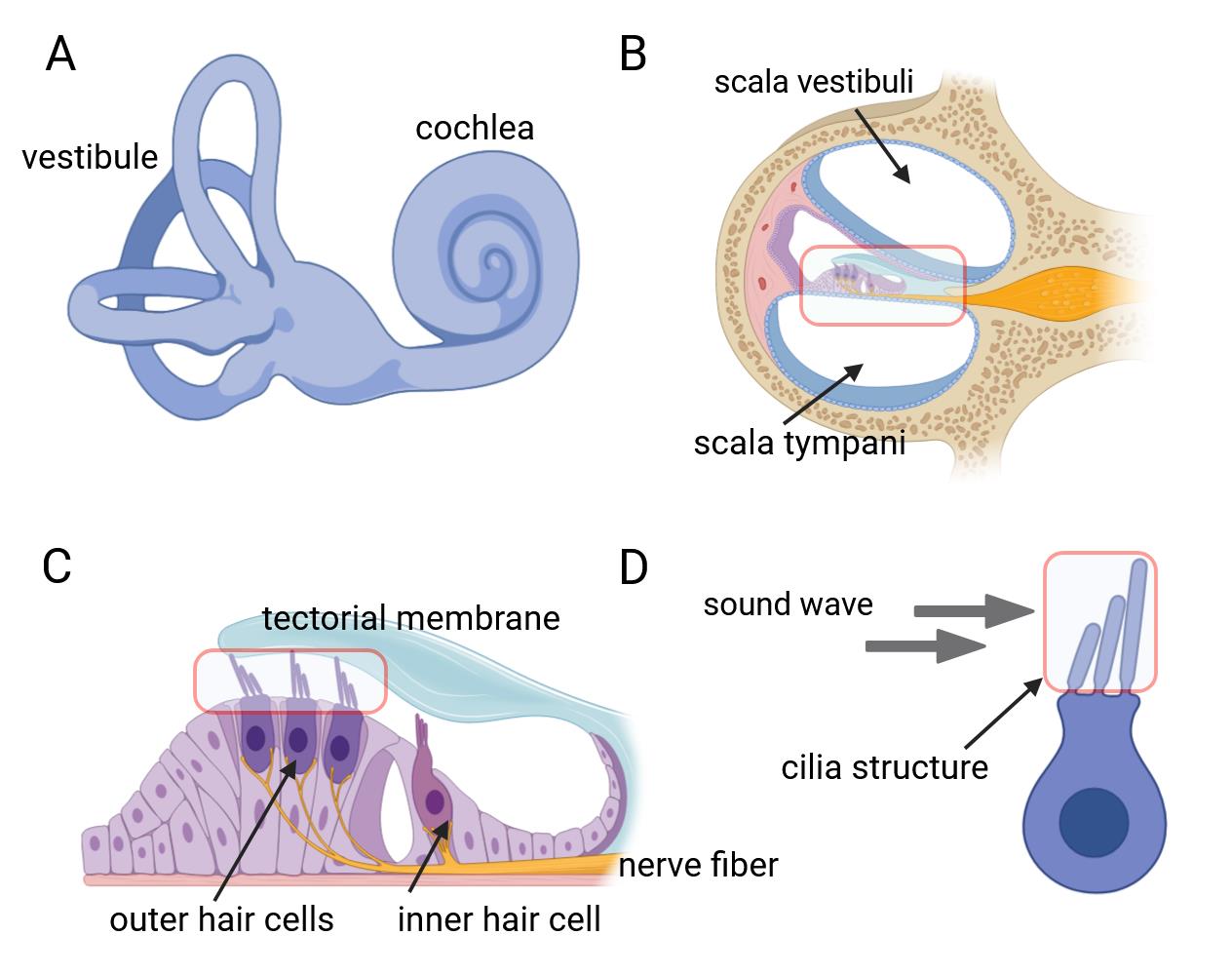
Figure 1. Inner ear anatomy. A, B) The cochlea and a close-up of a single turn cross-section, revealing the organ of Corti. C) The organ of Corti contains mechanosensitive hair cells, which lie beneath the tectorial membrane. D) Hair bundles composed of actin-filled stereocilia arranged in a staircase configuration.
Hair cells exhibit a unique form of planar cell polarity, with their stereocilia bundles oriented toward the peripheral region of the cochlear duct, characterized by a distinct alignment [10]. In this process, several proteins have to be targeted to specific cellular locations to signal the directionality in the cells and to build the specific polarity structure that is unique for the maintenance of the mechanotransduction apparatuses of the sensory hair cells [11–13]. Immunohistochemical techniques are employed to discern hair cell types and precisely locate native proteins contributing to cellular architecture and mechanotransduction capabilities. Furthermore, confocal microscopy enables precise visualization of the ultrastructural features and properties of cochlear lesions, thereby facilitating comprehension of auditory physiological and pathological dynamics. This innovative experimental methodology is extensively employed in fundamental research on auditory sensory cells within the cochlea. We hereby propose a comprehensive protocol designed for the meticulous examination of the mouse auditory organ, encompassing its anatomical structure, immunohistochemical staining procedures, and advanced confocal imaging techniques.
Materials and reagents
Biological materials
1. Rab11a conditional knock-out alleles, Vangl2-Looptail mice (The Jackson Laboratory, catalog number: 000220)
Reagents
1. Phosphate-buffered saline (PBS) (GENOM, catalog number: GNM20012-5)
2. Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: 158127)
3. Triton X-100 (Sigma, catalog number: 9002-93-1)
4. Normal donkey serum (Merck, Millipore, catalog number: S30)
5. Primary antibodies:
a. Rab11a, 1:200 (Cell Signaling Technology, catalog number: 2413)
b. γ-tubulin, 1:200 (Sigma, catalog number: T6557)
c. Arl13b, 1:1500 (Tamara Caspary, Emory University, Atlanta, GA)
d. Vangl2, 1:200 (R&D Systems, catalog number: AF4815)
e. Fz3, 1:500 (gift from Jeremy Nathans, Johns Hopkins University, Baltimore, MD)
f. LGN, 1:200 (gift from Fumio Matsuzaki, RIKEN)
g. β-Spectrin, 1:200 (BD Transduction Laboratories, catalog number: 612562)
h. MyosinVIIa, 1:200 (Proteus Bioscience Inc, catalog number: 25-6790)
i. Radixin, 1:100 (Abcam, catalog number: ab52495)
j. E-Cadherin, 1:200 (Invitrogen, catalog number: 13-1700)
6. Secondary antibodies:
a. Alexa Fluor® 488 AffiniPure donkey anti-mouse IgG (H+L), 1:1,000 (Jackson Immuno-Research Laboratories, catalog number: 715-545-151)
b. Rhodamine RedTM-X AffiniPure donkey anti-mouse IgG (H+L), 1:1,000 (Jackson Immuno-Research Laboratories, catalog number:715-295-151)
c. Alexa Fluor® 647 AffiniPure donkey anti-mouse IgG (H+L), 1:1,000 (Jackson Immuno-Research Laboratories, catalog number:715-605-151)
7. Phalloidin, 1:1,000 (Sigma-Aldrich, catalog number: P5282)
8. Mounting medium (Vectashield Antifade Medium, catalog number: H-1200)
9. Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S3014)
10. Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541)
11. Sodium phosphate dibasic heptahydrate (NaH2PO4·7H2O) (Sigma-Aldrich, catalog number: S9390)
12. Potassium dihydrogen phosphate (KH2PO4) (Sigma-Aldrich, catalog number: P9791)
Solutions
1. 1× PBS (see Recipes)
2. 4% PFA (see Recipes)
Recipes
1. 1× PBS
| Reagent | Final concentration | Amount |
|---|---|---|
| Sodium chloride | n/a | 8 g |
| Potassium chloride | n/a | 200 mg |
| Sodium phosphate dibasic heptahydrate | n/a | 1.44 g |
| Potassium dihydrogen phosphate | n/a | 240 mg |
| H2O | n/a | 1,000 mL |
| Total | n/a | 1,000 mL |
Adjust pH to 7.4 with HCl and store it at room temperature.
2. 4% PFA
| Reagent | Final concentration | Amount |
|---|---|---|
| Paraformaldehyde (powder) | n/a | 4 g |
| 1× PBS | n/a | 100 mL |
| Total | 4% | 100 mL |
Store paraformaldehyde powder at 4 °C. Heat while stirring under the chemical hood at approximately 60 °C. When the solution is transparent, let it cool down and then filter with Whatman paper to remove undissolved particles. Freeze PFA aliquots in 50 mL tubes at -20 °C. Remember to wear suitable personal protective equipment.
Equipment
1. Dissection microscope (BT, catalog number: BTS-300)
2. Large forceps (Fine Science Tools, catalog number: 11026-15)
3. Fine forceps (Fine Science Tools, catalog number: 11252-00)
4. Very fine forceps (Fine Science Tools, catalog number: 11200-14)
5. Small scissors (Fine Science Tools, catalog number: 91460-11)
6. Confocal microscope (Carl Zeiss Microscopy, model: LSM 510, AxioObserver)
7. 48-well plate (Corning, catalog number: 3548)
8. Petri dish (BD Biosciences, Falcon®, catalog number: 351006)
Software and datasets
1. ImageJ software (http://rsb.info.nih.gov/ij)
Procedure
文章信息
稿件历史记录
提交日期: Sep 15, 2024
接收日期: Nov 28, 2024
在线发布日期: Dec 10, 2024
出版日期: Jan 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Chen, C., Chen, B., Qian, X., Sun, H., Fu, X. and Ren, D. (2025). Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice. Bio-protocol 15(2): e5167. DOI: 10.21769/BioProtoc.5167.
- Knapp, L., Sun, H., Wang, Y. M., Chen, B. J., Lin, X., Gao, N., Chen, P. and Ren, D. (2023). Rab11a Is Essential for the Development and Integrity of the Stereocilia and Kinocilia in the Mammalian Organ of Corti. eNeuro. 10(6): ENEURO.0420–22.2023.
分类
细胞生物学 > 组织分析 > 组织分离
神经科学 > 基础技术 > 组织解剖
细胞生物学 > 细胞结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link