- EN - English
- CN - 中文
FlashTag-mediated Labeling for Intraventricular Macrophages in the Embryonic Brain
基于FlashTag的胚胎脑室内巨噬细胞标记方法
发布: 2025年01月20日第15卷第2期 DOI: 10.21769/BioProtoc.5166 浏览次数: 2223
评审: Miao HeKeiko MorimotoFereshteh Azedi

相关实验方案

研究免疫调控血管功能的新实验方法:小鼠主动脉与T淋巴细胞或巨噬细胞的共培养
Taylor C. Kress [...] Eric J. Belin de Chantemèle
2025年09月05日 3543 阅读
Abstract
The fate mapping technique is essential for understanding how cells differentiate and organize into complex structures. Various methods are used in fate mapping, including dye injections, genetic labeling (e.g., Cre-lox recombination systems), and molecular markers to label cells and track their progeny. One such method, the FlashTag system, was originally developed to label neural progenitors. This technique involves injecting carboxyfluorescein diacetate succinimidyl ester (CFSE) into the lateral ventricles of mouse embryos, relying on the direct uptake of dye by cells. The injection of CFSE into the lateral ventricle allows for the pulse labeling of mitotic (M-phase) neural progenitors in the ventricular zone and their progeny throughout the brain. This approach enables us to trace the future locations and differentiation paths of neural progenitors. In our previous study, we adapted this method to selectively label central nervous system–associated macrophages (CAMs) in the lateral ventricle by using a lower concentration of CFSE compared to the original protocol. Microglia, the brain's immune cells, which play pivotal roles in both physiological and pathological contexts, begin colonizing the brain around embryonic day (E) 9.5 in mice, with their population expanding as development progresses. The modified FlashTag technique allowed us to trace the fate of intraventricular CAMs, revealing that certain populations of microglia are derived from these cells. The optimized approach offers deeper insights into the developmental trajectories of microglia. This protocol outlines the modified FlashTag method for labeling intraventricular CAMs, detailing the CFSE injection procedure, evaluation of CFSE dilution, and preparation of tissue for immunohistochemistry.
Key features
• This protocol builds upon the method developed by Govindan et al. and extends its application to intraventricular CAMs.
• This protocol allows for the cell fate tracking of intraventricular CAMs within 24 h.
• This protocol requires the technique of intraventricular injection of CFSE into embryonic brains.
Keywords: Microglia (小胶质细胞)Graphical overview
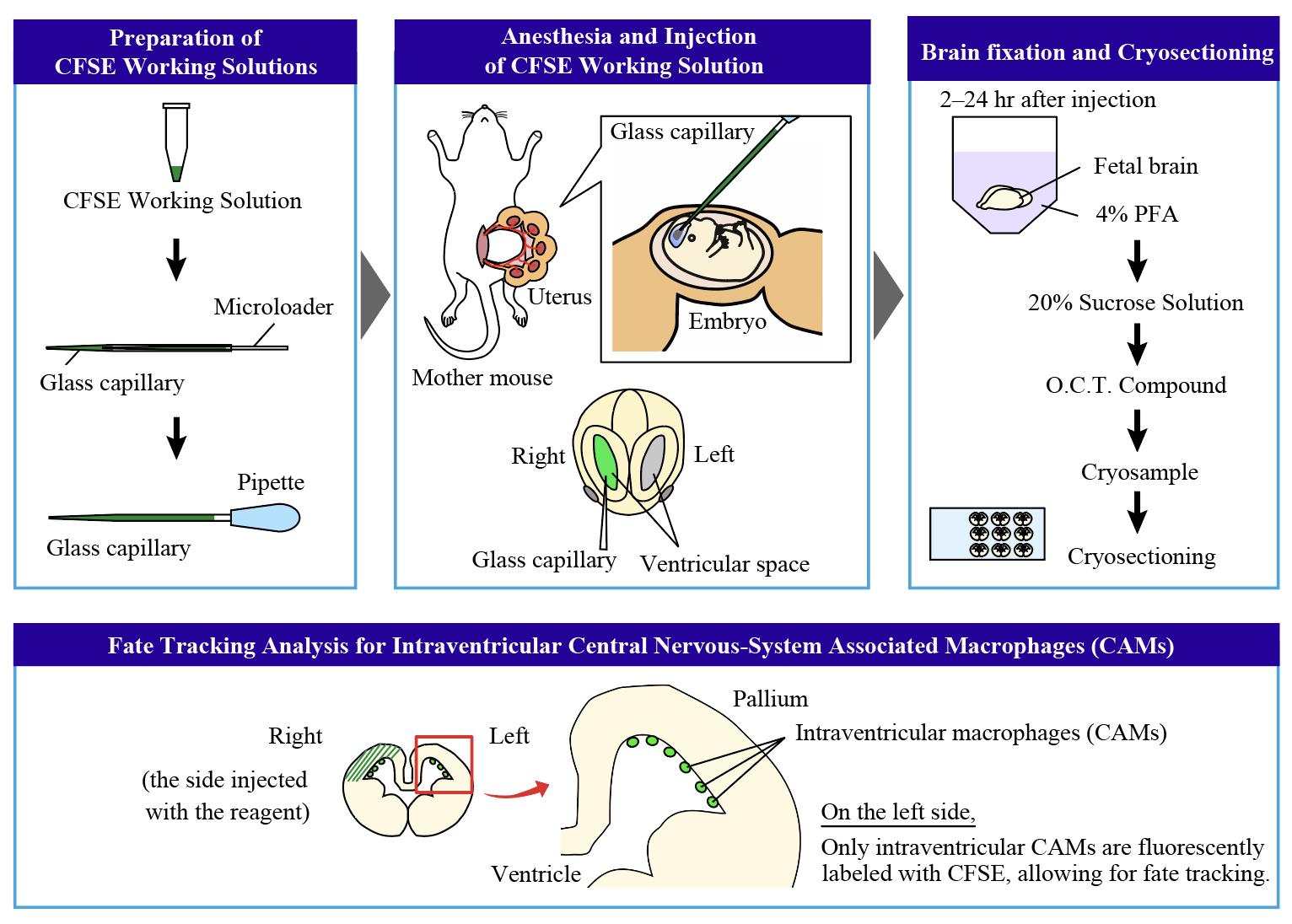
FlashTag-mediated labeling for intraventricular macrophages
Background
The fate mapping technique is crucial for deciphering how cells differentiate and organize into complex structures. Various methods, such as dye injections, Cre-lox genetic labeling, and molecular markers, are used to track the progeny of cells. One of the earliest and most widely used techniques is BrdU (5-bromo-2'-deoxyuridine) birth dating, which labels newborn neural lineage cells at a specific time point [1,2]. BrdU is incorporated into cells during the S phase of DNA synthesis. When administered to pregnant dams either via intraperitoneal injection or drinking water, it leads to systemic labeling of all S-phase cells in the embryo. This results in no spatial restriction, meaning all cells born across all regions are labeled. Another technique, in utero electroporation, is used to label newborn neural progenitors. In this method, a plasmid encoding a reporter protein is injected into the lateral ventricle of an embryonic mouse, and electrical pulses are applied to facilitate its entry into the cells [3,4]. This method primarily targets M-phase cells but can also label S-phase cells, allowing for the labeling of a wide range of unsynchronized progenitors. However, the detection of labeled cells only occurs after the reporter protein is expressed, typically about 10 h later, making it challenging to study immediate events post-transduction [5].
To overcome the limitations of traditional birth-dating techniques, the FlashTag method was developed to track isochronic cohorts of ventricular zone (VZ)-born cells in the developing central nervous system (CNS) [6,7]. This method uses carboxyfluorescein diacetate succinimidyl ester (CFSE), a dye that fluoresces only after being processed within the cell. It allows for the specific labeling of M-phase neural progenitors lining the VZ, with intracellular fluorescence detectable within 20 min of injection. In the developing pallium, the labeling window is narrow, restricted to 1–2 h post-injection [7].
Notably, the FlashTag method can be optimized to label CNS-associated macrophages (CAMs), which reside in the ventricular space. Our previous research showed that intraventricular CAMs, which frequently infiltrate the developing pallium at embryonic day 12.5 (E12.5), differentiate into microglia after their infiltration in mice [8]. Microglia, the immune cells of the CNS, play essential roles throughout various life stages. For example, microglia shape neuronal circuits by pruning synapses and maintain homeostasis by clearing apoptotic cells and debris in the postnatal and adult brain, while contributing to neurogenesis by regulating differentiation and cell number [9-13]. Genetic fate mapping studies in mice have shown that microglia and CAMs share a common origin from erythromyeloid progenitors (EMPs), which are generated in the yolk sac between E7.5 and E8.5 and colonize the brain around E9.5 [14,15].
In our previous study, we optimized the FlashTag method by using a lower concentration of CFSE, enabling us to trace the fate of intraventricular CAMs. We discovered that certain microglia populations originate from these CAMs, which infiltrate the pallium around E12.5. This protocol is particularly useful for tracking the fate and behavior of intraventricular CAMs within a limited time window.
Materials and reagents
Biological materials
1. ICR mice (purchased from Japan SLC)
Reagents
1. CellTraceTM CFSE Cell Proliferation kit (for flow cytometry) (Thermo Fisher Scientific, catalog number: C34554)
2. Fast Green FCF (FUJIFILM Wako Pure Chemical Corp., catalog number: 061-00031)
3. Otsuka normal saline (Otsuka Pharmaceutical Factory, Inc., catalog number: 035-081517)
4. Sodium azide (FUJIFILM Wako Pure Chemical Corp., catalog number: 195-11091)
5. NaCl (sodium chloride) (FUJIFILM Wako Pure Chemical Corp., catalog number: 191-01665)
6. KCl (potassium chloride) (Sigma-Aldrich, catalog number: 24-4290-5)
7. Na2HPO4-12H2O (FUJIFILM Wako Pure Chemical Corp., catalog number: 196-02835)
8. NaH2PO4-2H2O (FUJIFILM Wako Pure Chemical Corp., catalog number: 192-02815)
9. KH2PO4 (FUJIFILM Wako Pure Chemical Corp., catalog number: 167-04241)
10. HCl (Sigma-Aldrich, catalog number: 13-1640-5)
11. NaOH (FUJIFILM Wako Pure Chemical Corp., catalog number: 194-18865)
12. Triton X-100 (Thermo Scientific, catalog number: A16046-0F)
13. Bovine serum albumin (Sigma-Aldrich, catalog number: A9418-50G)
14. Tissue-Tek O.C.T. compound (Sakura Finetek Japan Co., Ltd., catalog number: 4583)
15. Goat anti-CD206 pAb (1:300) (R&D systems, Minneapolis, catalog number: AF2535, RRID: AB_2063012)
16. Mouse anti-FITC mAb (1:400) (BioLegend, catalog number: 408301, RRID: AB_528900)
17. Rabbit anti-IBA1 pAb (1:1000) (FUJIFILM Wako Pure Chemical Corp., catalog number: 019-19741, RRID: AB_839504)
18. Rabbit anti-P2RY12 pAb (1:500) (AnaSpec, catalog number: 55043A, RRID: AB_2298886)
19. Donkey anti-Mouse IgG (H+L) highly cross-adsorbed secondary antibody, Alexa FluorTM 488 (1:1000) (Thermo Fisher Scientific, catalog number: A-21202, RRID: AB_141607)
20. Donkey anti-Rabbit IgG (H+L) highly cross-adsorbed secondary antibody, Alexa FluorTM 546 (1:1000) (Thermo Fisher Scientific, catalog number: A-10040, RRID: AB_2534016)
21. Donkey anti-Rabbit IgG (H+L) highly cross-adsorbed secondary antibody, Alexa FluorTM 647 (1:1000) (Thermo Fisher Scientific, catalog number: A-31573, RRID: AB_2536183)
22. Donkey anti-Goat IgG (H+L) cross-adsorbed secondary antibody, Alexa FluorTM 546 (1:1000) (Thermo Fisher Scientific, catalog number: A-11056, RRID: AB_2534103)
23. Donkey anti-Goat IgG (H+L) cross-adsorbed secondary antibody, Alexa FluorTM 647 (1:1000) (Thermo Fisher Scientific, catalog number: A-21447, RRID: AB_2535864)
24. Paraformaldehyde (Sigma-Aldrich, Merck, catalog number: 818715)
25. Sucrose (FUJIFILM Wako Pure Chemical Corp., catalog number: 190-00015)
26. Medetomidine hydrochloride (Nippon Zenyaku Kogyo Co., Ltd., catalog number: N.A.)
27. Midazolam (FUJIFILM Wako Pure Chemical Corp., catalog number: 135-13791)
28. Butorphanol tartrate (Meiji Animal Health Co., Ltd., catalog number: N.A.)
29. Atipamezole hydrochloride (Kyoritsu Seiyaku Corporation., catalog number: N.A.)
30. Fluoromount-G(R) (Cosmo Bio Co Ltd., catalog number: 0100-01)
31. 70% ethanol
Solutions
1. CFSE stock solution (see Recipes)
2. 0.3% Fast Green solution (see Recipes)
3. 10× PBS, pH 7.4 (see Recipes)
4. 1× PBS sterile, pH 7.4 (see Recipes)
5. 1× PBS with sodium azide, pH 7.4 (see Recipes)
6. Sodium azide solution (1% (w/v)) (see Recipes)
7. 2.5% CFSE working solution (see Recipes)
8. 5% CFSE working solution (see Recipes)
9. 10% CFSE working solution (see Recipes)
10. Three types of mixed anesthetic agents (see Recipes)
11. Anesthetic antagonist solution (see Recipes)
12. 0.2M phosphate buffer, pH 7.4 (see Recipes)
13. 8% PFA solution, pH 7.4 (see Recipes)
14. 4% PFA solution, pH 7.4 (see Recipes)
15. 20% sucrose solution (see Recipes)
16. Antibody diluent solution (see Recipes)
Recipes
1. CFSE stock solution (8 μL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CellTraceTM CFSE (#C34554A) | 6.25 mg/mL | 50 μg |
| DMSO (attached in the kit; thaw if frozen) | n/a | 8 μL |
| Total | n/a | 8 μL |
CFSE stock solution should be stored at -30 °C.
2. 0.3% Fast Green solution (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Fast Green FCF | 0.3% (w/v) | 0.03 g |
| H2O (MilliQ water) | n/a | 10 mL |
| Total | n/a | 10 mL |
0.3% Fast Green solution should be filtered through Millex-GV 0.22 μm filter and then stored in a 1.5 mL tube at 4 °C.
3. 10× PBS, pH 7.4 (2 L)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | n/a | 160 g |
| KCl | n/a | 4 g |
| Na2HPO4-12H2O | n/a | 58 g |
| KH2PO4 | n/a | 4 g |
| H2O (MilliQ water) | n/a | see note* |
| Total | n/a | 2 L |
*Note: Allow the solution to mix completely and adjust the pH of the solution to 7.4 by adding 1M HCl and 1M NaOH while measuring with a pH meter. Then, bring the volume up to 2 L with MilliQ water.
4. 1× PBS sterile, pH 7.4 (500 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 10× PBS, pH 7.4 (Recipe 3) | n/a | 50 mL |
| H2O (MilliQ water) | n/a | 450 mL |
| Total | n/a | 500 mL see note* |
*Note: Confirm that the pH of the solution is 7.4 using a pH meter. If it deviates, adjust it by adding 1M HCl or 1M NaOH. Then, bring the volume up to 500 mL with MilliQ water. The solution should be autoclaved for 20 min at 121 °C.
5. 1× PBS with sodium azide, pH 7.4 (500 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1× PBS sterile, pH 7.4 (Recipe 4) | n/a | 500 mL |
| Sodium azide solution (1% w/v) (Recipe 6) | 0.002% (w/v) | 1 mL |
| Total | n/a | 501 mL |
6. Sodium azide solution (1% w/v) (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sodium azide | 1 g | n/a |
| H2O (MilliQ water) | n/a | 100 mL |
| Total | n/a | n/a |
The prepared solution should be stored at room temperature (RT).
7. 2.5% CFSE working solution (10 μL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CFSE stock solution (Recipe 1) | 2.5% (w/v) (156 μg/mL) | 0.25 μL |
| 1× PBS sterile, pH 7.4 (Recipe 4) | n/a | 8.75 μL |
| Fast Green solution (0.3% w/v) (Recipe 2) | 0.03% (w/v) | 1 μL |
| Total | n/a | 10 μL |
8. 5% CFSE working solution (10 μL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CFSE stock solution (Recipe 1) | 5% (w/v) (313 μg/mL) | 0.5 μL |
| 1× PBS sterile, pH 7.4 (Recipe 4) | n/a | 8.5 μL |
| Fast Green solution (0.3% w/v) (Recipe 2) | 0.03% (w/v) | 1 μL |
| Total | n/a | 10 µL |
9. 10% CFSE working solution (10 μL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CFSE stock solution (Recipe 1) | 10% (w/v) (625 µg/ml) | 1 μL |
| 1× PBS sterile, pH 7.4 (Recipe 4) | n/a | 8 μL |
Fast Green Solution (0.3% w/v) (Recipe 2) | 0.03% (w/v) | 1 μL |
| Total | n/a | 10 µL |
10. Three types of mixed anesthetic agents (35 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Midazolam | n/a | 40 mg |
| HCl (0.1M) | n/a | 2 mL see note* |
Medetomidine hydrochloride (1.0 mg/mL) | n/a | 3 mL |
| Butorphanol tartrate (5.0 mg/mL) | n/a | 10 mL |
| Otsuka normal saline | n/a | 20 mL |
| Total | n/a | 35 mL |
*Note: Midazolam needs to be diluted first using HCI (0.1M). After that, other reagents should be added.
Aliquot the prepared solution into 2 mL portions and store at 4 °C. Only the tube being used is stored at RT.
11. Anesthetic antagonist solution (20 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
Atipamezole hydrochloride (5.0 mg/mL) | n/a | 0.3 mL |
| Otsuka normal saline | n/a | 19.7 mL |
| Total | n/a | 20 mL |
The prepared solution is aliquoted into 2 ml portions and stored at 4 °C. Only the tube being used is stored at RT.
12. 0.2 M phosphate buffer, pH 7.4 (1 L)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4-12H2O | n/a | 58 g |
| NaH2PO4-2H2O | n/a | 5.9 g |
| H2O (MilliQ water) | n/a | see note* |
| Total | n/a | 1 L |
*Note: Allow the solution to mix completely and adjust the pH of the solution to 7.4 by adding 1M HCl and 1M NaOH while measuring with a pH meter. Then, bring the volume up to 1 L with MilliQ water.
13. 8% PFA solution, pH 7.4 (400 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Paraformaldehyde | 8% (w/v) | 32 g |
| H2O (MilliQ water) | n/a | 365 mL see note* |
| 10N NaOH | n/a | 90 μL see note** |
| H2O (MilliQ water) | n/a | see note** |
| Total | n/a | 400 mL |
*Note: Add 32 g of paraformaldehyde to 365 mL of MilliQ water and dissolve it by heating it with a hot stirrer.
**Note: Add 90 μL of 10N NaOH at a temperature higher than 60 °C. When the cloudiness disappears and the solution becomes transparent, MilliQ water should be added. Confirm that the pH of the solution is 7.4 using a pH meter. If it deviates, adjust it by adding 1M HCl or 1M NaOH. Then, bring the volume up to 400 mL with MilliQ water and filter the solution with filter paper.
The prepared solution should be stored at 4 °C.
14. 4% PFA solution, pH 7.4 (200 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 8% PFA solution, pH 7.4 (Recipe 13) | 4% (w/v) | 100 mL |
| 0.2M phosphate buffer, pH 7.4 (Recipe 12) | n/a | 100 mL |
| Total | n/a | 200 mL |
The prepared solution should be stored at 4 °C.
15. 20% sucrose solution (250 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1×PBS with sodium azide, pH 7.4 (Recipe 5) | n/a | 220 mL |
| Sucrose | 20% | 50 g |
| Total | n/a | 250 mL |
The prepared solution should be stored at 4 °C.
16. Antibody diluent solution (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1× PBS sterile, pH 7.4 (Recipe 4) | n/a | 100 mL |
| Triton X-100 | 0.1% (v/v) | 100 μL |
| Sodium azide | 0.1% (w/v) | 0.1 g |
| Bovine serum albumin | 3 mg/mL | 0.3 g |
| Total | n/a | n/a |
Filter the solution using Millex-HV 0.45 μm filter and store at 4 °C.
Laboratory supplies
1. Glass capillary with filament (NARISHIGE Group, catalog number: GD-1)
2. Eppendorf microloader (Merck, catalog number: EP5242956003)
3. Aspirator tube assembly (Drummondo, catalog number: 2-040-000)
4. Dropper silicone rubber for 2 mL (AZ ONE, catalog number: 6-356-02)
5. Heating pad (Koizumi, catalog number: N.A.)
6. Flat-bottom micro tube 1.5 mL large box of 500 × 20 bags (BIO-BIK, catalog number: CF-0150)
7. Corning 100 mm non-treated culture dish (Corning, catalog number: 430591)
8. Transfer pipette 2.3 mL (BM Equipment Co., Ltd., catalog number: 262-20S)
9. Extra fine Graefe forceps (Fine Science Tools, catalog number: 11151-10)
10. Fine Iris scissors (Fine Science Tools, catalog number: 14094-11)
11. Dumont #55 fine forceps (Fine Science Tools, catalog number: 11255-20)
12. Paper filter (As ONE, catalog number: 65-0426-67)
13. Millex-GV 0.22 μm filter (Millipore, catalog number: SLGVJ13SL)
14. Millex-HV 0.45 μm filter (Millipore, catalog number: SLHVR33RS)
15. Nipro Flomax hypodermic 26G (S.B) needle (Nipro, catalog number: 01046)
16. Terumo 1 mL syringe (Terumo, catalog number: SS-01T)
17. Nylon suture needles with thread (BEAR Medic Corporation, catalog number: SP15A05H-45)
18. MAS coat slide glass (Matsunami Glass Ind., Ltd., catalog number: SMAS-01)
19. NEO micro cover glass (Matsunami Glass Ind., Ltd., catalog number: C024501)
Equipment
1. Magnetic glass microelectrode horizontal puller (Narishige, model: PN-30)
2. Micro grinder (Narishige, model: EG-400)
3. e-HeatingBucket (Miracle beads bath, TAITEC Corp, model: BMB-17)
4. Confocal microscopy (Nikon, model: AXR)
5. Confocal microscopy (Nikon, model: TiE-A1R)
6. Cryostat (Leica Biosystems, model: CM1520)
Procedure
文章信息
稿件历史记录
提交日期: Sep 22, 2024
接收日期: Nov 28, 2024
在线发布日期: Dec 12, 2024
出版日期: Jan 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Asai, H., Ono, M., Miyata, T. and Hattori, Y. (2025). FlashTag-mediated Labeling for Intraventricular Macrophages in the Embryonic Brain. Bio-protocol 15(2): e5166. DOI: 10.21769/BioProtoc.5166.
分类
神经科学 > 发育 > 神经元
发育生物学 > 细胞生长和命运决定 > 分化
免疫学 > 免疫细胞功能 > 巨噬细胞
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link