- EN - English
- CN - 中文
Mouse-derived Synaptosomes Trypsin Cleavage Assay to Characterize Synaptic Protein Sub-localization
基于小鼠突触体的胰蛋白酶切割实验解析突触蛋白亚细胞定位
发布: 2025年01月20日第15卷第2期 DOI: 10.21769/BioProtoc.5164 浏览次数: 1699
评审: Marion HoggXiaoliang ZhaoAnonymous reviewer(s)
Abstract
Neurons communicate through neurotransmission at highly specialized junctions called synapses. Each neuron forms numerous synaptic connections, consisting of presynaptic and postsynaptic terminals. Upon the arrival of an action potential, neurotransmitters are released from the presynaptic site and diffuse across the synaptic cleft to bind specialized receptors at the postsynaptic terminal. This process is tightly regulated by several proteins at both presynaptic and postsynaptic sites. The localization, abundance, and function of these proteins are essential for productive neurotransmission and are often affected in neurological and neurodegenerative disorders. Here, we outline a method for purifying mouse synaptosomes and using limited tryptic digestion to assess the subcellular localization of synaptic proteins. During synaptosomes purification, presynaptic terminals reseal and are protected from proteolysis, while postsynaptic proteins remain susceptible to tryptic cleavage. These changes can easily be evaluated by western blot analysis. This approach offers a straightforward and reliable method to evaluate the subcellular localization of synaptic proteins based on their proteolytic sensitivity, providing valuable insights into synaptic physiology and pathology.
Key features
• Builds upon the method developed by Boyken et al. [1] and introduces the use of isolated mouse synaptosomes to assess synaptic protein sub-localization.
• Limited tryptic digestion differentiates between presynaptic and postsynaptic proteins based on proteolytic sensitivity.
• Requires standard biochemical reagents and western blotting equipment and can be completed in two/three days, including synaptosome purification and western blot analysis.
Keywords: Synaptosomes (突触体)Graphical overview
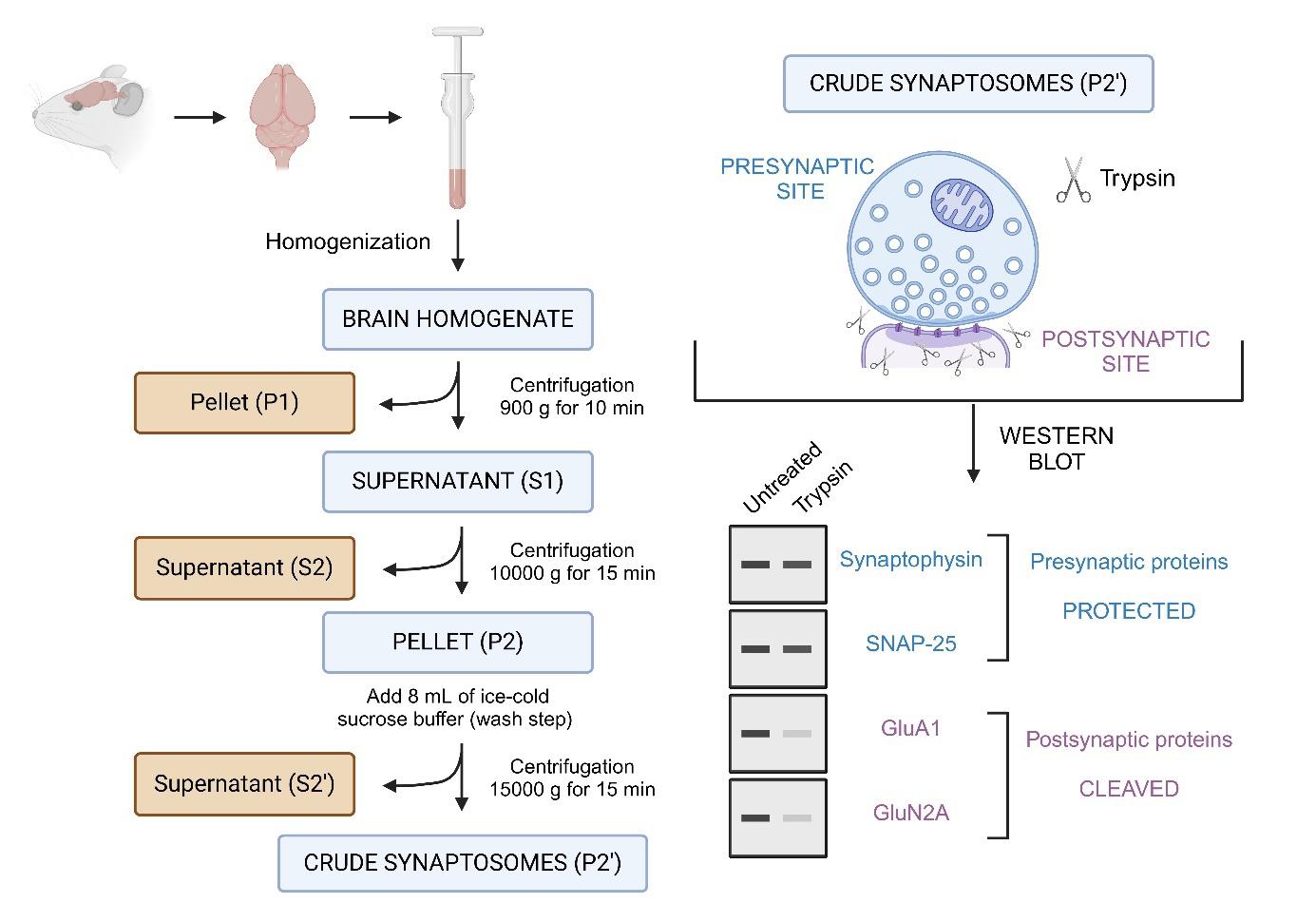 Overview of the synaptosomes trypsin cleavage assay. This protocol describes the isolation of synaptosomes from mouse brain tissue, followed by limited trypsin digestion to assess the compartmental localization of synaptic proteins. Synaptosomes, which are isolated via differential centrifugation, consist of resealed presynaptic terminals that are protected from proteolysis, while the exposed postsynaptic compartments are accessible to trypsin and undergo proteolytic cleavage. Following digestion, samples are analyzed using SDS-PAGE and western blotting. Proteins of interest are probed using specific antibodies to determine whether they are presynaptic or postsynaptic. Presynaptic proteins (e.g., synaptophysin, SNAP-25) remain intact, while postsynaptic proteins (e.g., GluA1, GluN2A) are cleaved.
Overview of the synaptosomes trypsin cleavage assay. This protocol describes the isolation of synaptosomes from mouse brain tissue, followed by limited trypsin digestion to assess the compartmental localization of synaptic proteins. Synaptosomes, which are isolated via differential centrifugation, consist of resealed presynaptic terminals that are protected from proteolysis, while the exposed postsynaptic compartments are accessible to trypsin and undergo proteolytic cleavage. Following digestion, samples are analyzed using SDS-PAGE and western blotting. Proteins of interest are probed using specific antibodies to determine whether they are presynaptic or postsynaptic. Presynaptic proteins (e.g., synaptophysin, SNAP-25) remain intact, while postsynaptic proteins (e.g., GluA1, GluN2A) are cleaved.
Background
Neurotransmission is the fundamental process by which neurons communicate and relay information, enabling neuronal circuits to function effectively, thus governing higher-order functions such as memory formation and storage [2]. This communication occurs at specialized structures called synapses, which consist of presynaptic and postsynaptic terminals [2–5]. In the presynaptic neuron, upon the arrival of an action potential, neurotransmitter-containing synaptic vesicles fuse with the presynaptic membrane, releasing their contents into the synaptic cleft [2–5]. These neurotransmitters diffuse across the cleft and bind to specialized receptors at the postsynaptic terminal, triggering the transmission of signals in the postsynaptic neuron [2–5]. This entire process is tightly regulated by a complex set of proteins [6–7]. Understanding the localization, abundance, and function of these proteins is essential for uncovering the mechanisms that regulate synaptic transmission, which can be altered in a variety of neurological and neurodegenerative disorders, such as Alzheimer's disease, Parkinson’s disease, and schizophrenia [8–9].
Several methodologies have been developed to study the protein composition of synapses [10]. Techniques such as immunohistochemistry and mass spectrometry have been widely used to map synaptic proteins [11–14]. Although these methods offer valuable insights into synaptic protein localization and abundance, they lack the resolution to distinguish between presynaptic and postsynaptic sites, which are separated by the synaptic cleft (approximately 20 nm wide). More recently, super-resolution microscopy has offered this level of resolution, but it often involves significant sample preparation time and is less suitable for high-throughput analysis [15–16]. To address some of these limitations and to isolate synaptic vesicle docking complexes, a method involving the use of synaptosomes combined with limited tryptic digestion has been developed by Boyken et al. [1]. This approach allows for the study of synaptic proteins in a more high-throughput and compartment-specific manner. Synaptosomes are partially resealed structures derived from isolated nerve terminals from mouse brains, in which presynaptic terminals remain sealed and intact while the postsynaptic terminal is left unprotected. By applying limited tryptic digestion to these synaptosomes, presynaptic proteins are protected from proteolysis, as they remain inaccessible to trypsin, while postsynaptic proteins are vulnerable to cleavage [1]. This method thus enables the distinction between presynaptic and postsynaptic proteins by western blot analysis, offering an efficient way to assess their localization.
To our knowledge, the protocol presented here has been used to study the molecular architecture of active zones [1], to confirm the postsynaptic localization of Synaptotagmin-3 [17], to assess the large postsynaptic pools of CALM [18] and intersectin1 (ITSN1) [19], and to confirm the postsynaptic localization of the total and phosphorylation forms of p85S6K over p70S6K, which instead appear to be mainly presynaptic [20]. Nevertheless, this protocol has the potential to be applied in various contexts in combination with mass spectrometry to enable a more precise understanding of the molecular components of synapses.
Materials and reagents
Biological materials
1. Mouse (Mus musculus, C57BL/6J) brain (preferably P60 or above)
Reagents
1. Sucrose (ROTH, catalog number: 9097.1)
2. HEPES (ROTH, catalog number: 6763.2)
3. Trypsin (Sigma-Aldrich, catalog number: T1005)
4. Bradford reagent (Sigma-Aldrich, catalog number: B6916)
5. Tris (ROTH, catalog number: AE15.6)
6. Glycerin (ROTH, catalog number: 3783.3)
7. SDS (ROTH, catalog number: 2326.2)
8. 2-Mercaptoethanol (Sigma Aldrich, catalog number: M3148)
9. Bromophenol blue (ROTH, catalog number: T116.1)
10. HCl (ROTH, catalog number: 4625.1)
11. Glycine (ROTH, catalog number: 3790.3)
12. Methanol (ROTH, catalog number: 8388.6)
13. Ponceau S (ROTH, catalog number: 5938.1)
14. Acetic acid (ROTH, catalog number: 3738.2)
15. Disodium hydrogen phosphate (Na2HPO4) (ROTH, catalog number: T876.1)
16. Potassium dihydrogen phosphate (KH2PO4) (ROTH, catalog number: 3904.1)
17. Sodium chloride (NaCl) (ROTH, catalog number: 3957.1)
18. Potassium chloride (KCl) (ROTH, catalog number: 6781.1)
19. Tween-20 (Polysorbat) (Fischer Scientific, catalog number: T/4206/60)
20. Intercept blocking buffer (LI-COR, catalog number: 927-60001)
21. ROTIPHORSE Gel 30 (37.5:1), ready-to-use acrylamide (ROTH, catalog number: 3029.1)
22. Ammonium peroxydisulfate (APS) (ROTH, catalog number: 9592.5)
23. TEMED (ROTH, catalog number: 2367.1)
24. Bovine serum albumin fraction V (BSA) (Sigma-Aldrich, catalog number: 1.12018.0100)
25. 2-propanol (isopropanol) (VWR, catalog number: 20842.323)
26. PageRuler prestained protein ladder (Thermo Scientific, catalog number: 26616)
27. Blotting paper (MACHEREY-NAGEL, catalog number: MN 827 B)
28. Nitrocellulose membrane (Amersham, catalog number: 10600004)
29. Anti-GluA1 antibody (Merk Millipore, catalog number: MAB2263)
30. Anti-GluA2 antibody (Merk Millipore, catalog number: MAB397)
31. Anti-GluN2A antibody (Cell signalling, catalog number: #4205)
32. Anti-GluN2B antibody (Cell signalling, catalog number: #4207)
33. Anti-Homer1 antibody (Synaptic Systems, catalog number: 160003)
34. Anti-Synaptotagmin1 antibody (SYT1) (Synaptic Systems, catalog number: 105103)
35. Anti-Synaptophysin1 antibody (SYP1) (Synaptic Systems, catalog number: 101011)
36. Anti-Snap25 antibody (Synaptic Systems, catalog number: 111011)
37. IRDye 800CW goat anti-Rabbit IgG secondary antibody (LI-COR, catalog number: 926-32211)
38. IRDye 680RD goat anti-Mouse IgG secondary antibody (LI-COR, catalog number: 926-68070)
Solutions
1. Sucrose buffer (see Recipes)
2. BSA solution for Bradford assay (see Recipes)
3. Trypsin solution (see Recipes)
4. 6× Loading sample buffer (LSB) (see Recipes)
5. 4× separating gel buffer (see Recipes)
6. 4× stacking gel buffer (see Recipes)
7. 10× running buffer (see Recipes)
8. 1× running buffer (see Recipes)
9. 10× transfer buffer (see Recipes)
10. 1× transfer buffer (see Recipes)
11. Ponceau S staining solution (see Recipes)
12. 10× PBS (see Recipes)
13. 1× PBS (see Recipes)
14. 1× PBST (see Recipes)
15. Blocking solution (see Recipes)
16. 10% APS solution (see Recipes)
Recipes
1. Sucrose buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sucrose | 0.32 M | 10.95 g |
| HEPES | 5 mM | 0.119 g |
| Water | n/a | 100 mL |
| Total | n/a | 100 mL |
Adjust the pH to 8.0 before reaching the final volume.
2. BSA stock solution for Bradford assay
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| BSA | 2 mg/mL | 2 mg |
| Sucrose buffer | n/a | 1 mL |
| Total | n/a | 1 mL |
3. Trypsin solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Trypsin | 0.1 mg/mL | 1 mg |
| Water | n/a | 10 mL |
| Total | n/a | 10 mL |
Store the solution on ice after preparation.
4. 6× loading sample buffer (LSB)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris pH 6.8 | 375 mM | 2.268 g |
| Glycerin | 50% | 25 mL |
| 2-mercaptoethanol | 10% | 5 mL |
| SDS | 10% | 5 g |
| Bromophenol blue | 0.03% | 15 mg |
| Total | n/a | 50 mL |
Heat the solution to 55 °C to dissolve the SDS, then add 2-mercaptoethanol at the end.
5. 4× Separating gel buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris pH 8.8 | 1.5 M | 90.86 g |
| SDS | 0.4% | 2 g |
| Water | n/a | 500 mL |
| Total | n/a | 500 mL |
Tris base pH level is adjusted to 8.8 using HCl. Add SDS at the end.
6. 4× Stacking gel buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris pH 6.8 | 0.5 M | 30.29 g |
| SDS | 0.4% | 1 g |
| Water | n/a | 250 mL |
| Total | n/a | 250 mL |
Tris base pH level is adjusted to 6.8 using HCl. Add SDS at the end.
7. 10× Running buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris pH 8.4 | 250 mM | 30.29 g |
| Glycine | 1.92 M | 144.13 g |
| SDS | 1% | 10 g |
| Water | n/a | 1,000 mL |
| Total | n/a | 1,000 mL |
8. 1× Running buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 10× Running buffer | 1× | 100 mL |
| Water | n/a | 900 mL |
| Total | n/a | 1,000 mL |
9. 10× Transfer buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris | 250 mM | 30.29 g |
| Glycine | 1.92 M | 144.13 g |
| Water | n/a | 1,000 mL |
| Total | n/a | 1,000 mL |
10. 1× Transfer buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 10× Transfer buffer | 1× | 100 mL |
| Methanol | 20% | 200 mL |
| Water | n/a | 700 mL |
| Total | n/a | 1,000 mL |
11. Ponceau S staining solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ponceau S | 0.5% | 1.25 g |
| Acetic acid | 1% | 2.5 mL |
| Water | n/a | 250 mL |
| Total | n/a | 250 mL |
12. 10× PBS
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4 | 100 mM | 11.49 g |
| KH2PO4 | 18 mM | 2.45 g |
| NaCl | 1.4 M | 82.82 g |
| KCl | 27 mM | 2.01 g |
| Water | n/a | 1,000 mL |
| Total | n/a | 1,000 mL |
13. 1× PBS
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 10× PBS | 1× | 100 mL |
| Water | n/a | 900 mL |
| Total | n/a | 1,000 mL |
14. 1× PBST
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 10× PBS | 1× | 100 mL |
| Tween-20 | 0.1% | 1 mL |
| Water | n/a | 900 mL |
| Total | n/a | 1,000 mL |
15. Blocking solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| LiCOR blocking solution | 50% | 50 mL |
| PBST | 50% | 50 mL |
| Total | n/a | 100 mL |
16. 10% APS solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| APS | 10% | 1 g |
| Water | n/a | 10 mL |
| Total | n/a | 10 mL |
Laboratory supplies
1. Falcon 15 mL centrifuge tube (CORNING, catalog number: 352096)
2. Falcon 50 mL centrifuge tube (CORNING, catalog number: 352070)
3. Ultra-high performance centrifuge tubes (VWR, 525-1085)
4. SurPhob tips EcoReload, 1,250 μL (Biozym, catalog number: VT0174)
5. SurPhob tips EcoReload, 200 μL (Biozym, catalog number: VT0144)
6. SurPhob tips EcoReload, 10 μL (Biozym, catalog number: VT0104)
7. Transferpette S pipette 100–1,000 μL (BRAND, catalog number: BR705880)
8. Transferpette S pipette 20–200 μL (BRAND, catalog number: BR705878)
9. Transferpette S pipette 0.1–2.5 μL (BRAND, catalog number: BR705869)
10. Reaction tubes 1.5 mL (Biozym, catalog number: 710310)
11. 96-well flat bottom plate (Anicrin, catalog number: M09600P0)
12. Ice buckets for cooling steps
Equipment
1. Water bath (Lauda, model: Hydro H 20 S)
2. Centrifuge (Eppendorf, model: 5910 Ri, rotor: FA-6x50)
3. Odyssey Fc imaging system (Li-COR Odyssey Fc, model: 2800)
4. Homogenizer (Heidolf, model: Hei-TORQUE core)
5. Tissue grind pestle (Kimble Chase, catalog number: 885481-0023)
6. Mini-Protean Tetra cell casting modules (Bio-Rad, catalog number: 1658050)
7. Mini-Protean Tetra cell 4-gel system (Bio-Rad, catalog number: 1658004)
8. Criterion Blotter with wire electrodes (Bio-Rad, catalog number: 1704071)
9. Thermal shaker (VWR, model: Thermal shake lite, catalog number: 460-0249P)
10. SpectroStar Nano V5.70 (BMG Labtech)
Software and datasets
Image studio (Li-COR, Version 5.2)
MARS data analysis software (BMG Labtech, Version 3.42)
Procedure
文章信息
稿件历史记录
提交日期: Oct 8, 2024
接收日期: Nov 21, 2024
在线发布日期: Dec 10, 2024
出版日期: Jan 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Shergill, J. K. and Azarnia Tehran, D. (2025). Mouse-derived Synaptosomes Trypsin Cleavage Assay to Characterize Synaptic Protein Sub-localization. Bio-protocol 15(2): e5164. DOI: 10.21769/BioProtoc.5164.
- Azarnia Tehran, D., Kochlamazashvili, G., Pampaloni, N. P., Sposini, S., Shergill, J. K., Lehmann, M., Pashkova, N., Schmidt, C., Löwe, D., Napieczynska, H., et al. (2022). Selective endocytosis of Ca 2+ -permeable AMPARs by the Alzheimer’s disease risk factor CALM bidirectionally controls synaptic plasticity. Sci Adv. 8(21): eabl5032. https://doi.org/10.1126/sciadv.abl5032
分类
神经科学 > 细胞机理 > 突触生理学
生物化学 > 蛋白质 > 分离和纯化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
提问指南
+ 问题描述
写下详细的问题描述,包括所有有助于他人回答您问题的信息(例如实验过程、条件和相关图像等)。
Share
Bluesky
X
Copy link