- EN - English
- CN - 中文
An Optimized Protocol for Simultaneous Propagation of Patient-derived Organoids and Matching CAFs
优化的患者来源类器官与匹配癌相关成纤维细胞同步培养方案
发布: 2025年01月20日第15卷第2期 DOI: 10.21769/BioProtoc.5160 浏览次数: 4425
评审: Anca Flavia Savulescuilgen Mender
Abstract
Recurrent hormone receptor-positive (HR+) breast cancer is a leading cause of cancer mortality in women. Recurrence and resistance to targeted therapies have been difficult to study due to the long clinical course of the disease, the complex nature of resistance, and the lack of clinically relevant model systems. Existing models are limited to a few HR+ cell lines, organoid models, and patient-derived xenograft models, all lacking components of the human tumor microenvironment. Furthermore, the low take rate and loss of estrogen receptor (ER) expression in patient-derived organoids (PDOs) has been challenging. Our protocol allows simultaneous isolation of PDOs and matching cancer-associated fibroblasts (CAFs) from primary and metastatic HR+ breast cancers. Importantly, our protocol has a higher take rate and enables long-term culturing of PDOs that retain ER expression. Our matching PDOs and CAFs will provide researchers with a new resource to study the influence of the tumor microenvironment on various aspects of cancer biology such as cell growth and drug resistance in HR+ breast cancer.
Key features
• Propagation of patient-derived organoids and matching cancer-associated fibroblasts from primary and metastatic hormone receptor (HR+) positive breast cancer.
• Optimized media for long-term culturing of HR+ organoids from primary tumors and bone metastasis.
• Co-culture model to assess the influence of the tumor stroma on breast cancer progression.
Keywords: Patient-derived organoids (患者来源类器官)Graphical overview
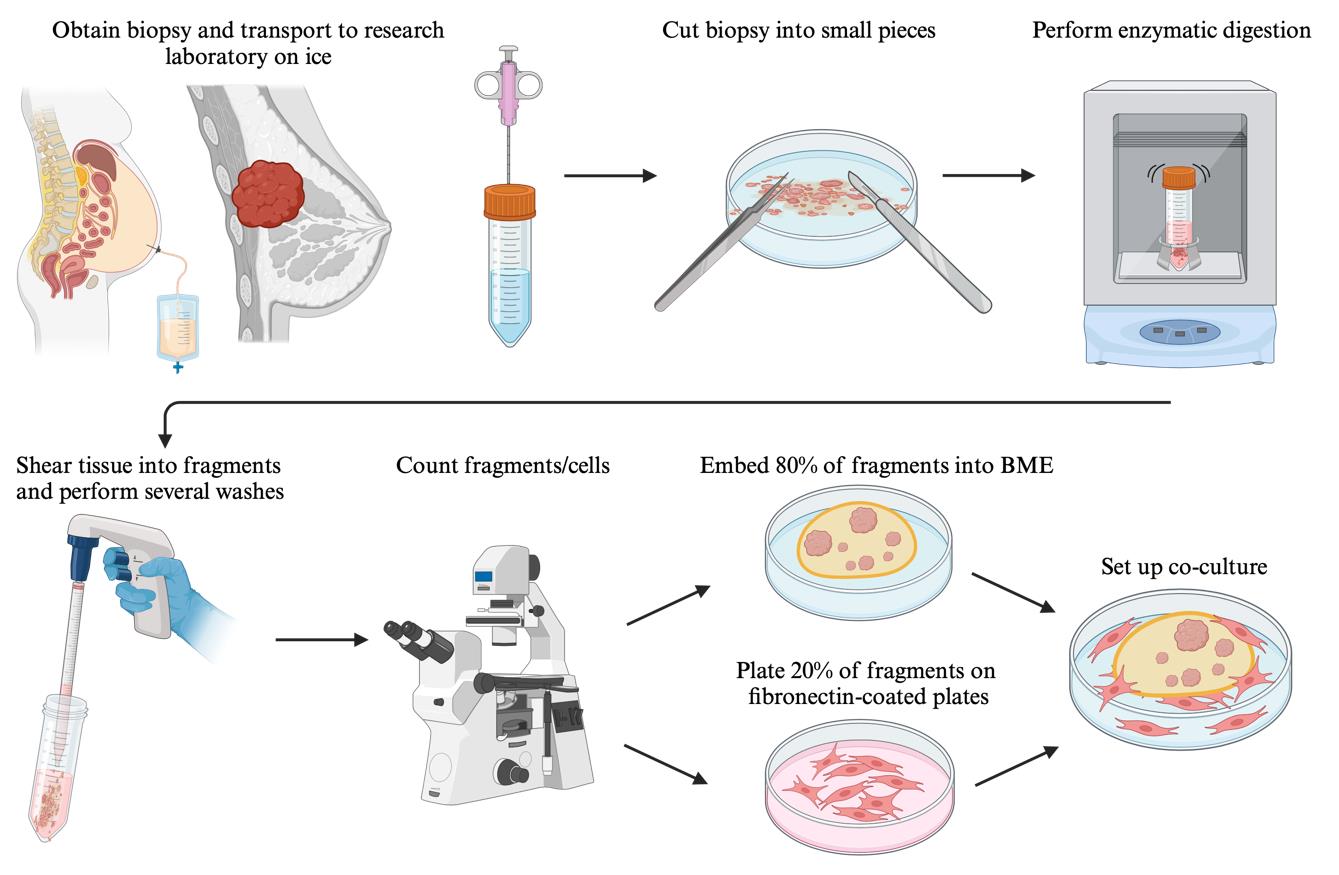
Graphical overview of the key steps for establishing patient-derived organoid cultures and matching cancer-associated fibroblasts from hormone receptor-positive breast cancer
Background
Breast cancer is the second leading cause of cancer-related mortality in women with over 600,000 deaths yearly. The majority of newly diagnosed breast cancers are hormone receptor-positive (HR+), expressing estrogen receptor (ER) with or without progesterone receptor (PR) [1]. These patients are treated with targeted therapies, such as endocrine therapies and CDK4/6 inhibitors [2]. Although HR+ breast cancers typically respond well to these therapies, approximately 30% of the patients will relapse. Unfortunately, recurrent HR+ breast tumors are usually metastatic and incurable. Besides tumor-intrinsic factors, the breast tumor microenvironment contributes to adaptive resistance [3]. Cancer-associated fibroblasts (CAFs) significantly impact tumor biology and are known to induce resistance to targeted therapies [4–9]. Despite this, CAFs are rarely incorporated into studies on breast cancer recurrence and resistance.
Patient-derived organoids (PDOs) are 3-dimensional multicellular clusters that are grown in basement membrane extract (BME). PDOs have self-renewal and self-organization capabilities and retain several features of the original tumor such as morphology and mutational landscape [10]. Compared to the traditional 2-dimensional cell cultures, PDOs capture intratumoral heterogeneity and are superior at predicting drug responses. To date, PDOs have been generated from several tumors, including breast cancer [11–13]. However, growing HR+ PDOs has been a challenge due to a low take rate (10%), overgrowth of normal breast epithelial cells, and the PDOs losing their HR expression and ceasing to grow after only a few passages [14,15]. In addition to PDOs, breast tumor organoids can be propagated from patient-derived xenografts (PDxO) [16,17]. Similar to the HR+ PDOs, HR+ PDxOs have a take rate of 9% for primary tumors and 16% for metastatic tumors. Currently, there are only a few models of metastatic HR+ breast cancer available [16,18]. Thus, there is an unmet need for novel models of HR+ primary and metastatic breast cancer that would advance our understanding of HR+ disease relapse and drug resistance.
We set out to develop patient-derived model systems for HR+ breast cancer that would also incorporate elements of the tumor microenvironment. Our optimized protocol allows for the efficient generation of HR+ PDOs from both primary and metastatic HR+ breast cancer with ~50% take rate. Importantly, we further optimized the media to support isolation and long-term passaging of HR+ bone metastasis that has been extremely difficult to grow. Our method also provides the advantage of reducing the cost and time by circumventing the necessity to passage through a mouse host. Furthermore, we isolate the matching CAFs that most protocols discard; thus, our optimized protocol offers the advantage of enabling the investigation of resistance mechanisms induced by the CAFs. Besides utilizing the patient-derived model for studying HR+ breast cancer and drug resistance [19–21], this model system can be applied to studying various aspects of tumor-stroma interactions.
Materials and reagents
Biological materials
1. Core needle biopsy, primary or metastatic breast cancer
2. Fine-needle aspiration (FNA), lymph node positive for tumor cells
3. Ascites, pleural, or peritoneal fluid
Reagents
1. Phosphate buffered saline (PBS) (Cytiva, catalog number: SH30028.FS)
2. Penicillin-streptomycin (Pen/Strep), 100× (Thermo Fisher, catalog number: 15140122)
3. Dispase-II solution (Sigma, catalog number: SCM133)
4. Collagenase type I (Sigma, catalog number: SCR103)
5. Human fibronectin solution (Sigma, catalog number: F0556)
6. Glucose solution, 200 g/L (Thermo Fisher, catalog number: A24940-01)
7. L-Glutamine solution, 200× (Thermo Fisher, catalog number: 25030-081)
8. HEPES solution, 1M (Sigma, catalog number: H0887-100ML)
9. Y-27632 2HCl, 10 mg (Selleckchem, catalog number: S1049)
10. Advanced DMEM/F12, 1× (Gibco, catalog number: 12634-010)
11. Primocin, 500 mg (InvivoGen, catalog number: ant-pm-05)
12. B27 supplement, 50× (Thermo Fisher, catalog number: 17504044)
13. Nicotinamide (Sigma, catalog number: N0636)
14. N-Acetyl-L-Cysteine (Sigma, catalog number: A9165)
15. A82-01 (Tocris, catalog number: 2939)
16. SB202190 (Selleckchem, catalog number: S1077)
17. 17β-Estradiol (Sigma, catalog number: 3301)
18. Hydrocortisone (Sigma, catalog number: H0888)
19. Human EGF (Thermo Fisher, catalog number: AF-100-15)
20. Human R-Spondin3 (Thermo Fisher, catalog number: 120-44)
21. Human noggin (Thermo Fisher, catalog number: 120-10C)
22. Human heregulin-β1 (Thermo Fisher, catalog number: 100-03)
23. Human FGF7 (Thermo Fisher, catalog number: 100-19)
24. Human FGF10 (Thermo Fisher, catalog number: 100-26)
25. Human CXCL12 (Thermo Fisher, catalog number: 300-28A)
26. Human IGF-I (Thermo Fisher, catalog number: 100-11)
27. Human osteopontin (Thermo Fisher, catalog number: 120-35)
28. Cultrex growth factor–reduced basement membrane extract type II (Fisher Scientific, catalog number: 353301002P)
29. Human fibroblast expansion basal medium, 1× (Thermo Fisher, catalog number: M106500)
30. Low serum growth supplement, 50× (Thermo Fisher, catalog number: S00310)
31. Fetal bovine serum (FBS) (R&D Systems, catalog number: S11550H)
32. Trypsin-EDTA, 0.25% (Thermo Fisher, catalog number: 25200056)
33. TrypLE Express, 1× (Thermo Fisher, catalog number: 12604013)
34. Dimethyl sulfoxide (DMSO) (Sigma, catalog number: D2650)
35. Corning cell recovery solution (Corning, catalog number: 354253)
36. CryoStor CS10 cryopreservation media (Sigma, catalog number: C2874)
37. Paraformaldehyde (PFA), 16%, EM grade (Electron Microscopy Sciences, catalog number: 15710-S)
38. HistoGelTM (Fisher Scientific, catalog number: 22-110-678)
39. Hematoxylin (Sigma, catalog number: MHS32-1L)
40. 10% formalin (Electron Microscopy Sciences, catalog number: 15740-04)
41. Bovine serum albumin (BSA) (Sigma, catalog number: A8806)
42. Goat serum (Thermo Fisher, catalog number: 16210064)
43. Triton X-100 (Sigma, catalog number: X100-500ML)
44. Cytoseal 60 (Fisher Scientific, catalog number: 23-244256)
45. Vectashield antifade mounting medium with DAPI (Fisher Scientific, catalog number: NC9524612)
46. Primary antibodies (Table 1)
Table 1. Primary antibodies for validation of ER status and characterization of CAFs
| Antibody | Host | Company | Catalog number |
|---|---|---|---|
| Estrogen receptor | rabbit | Abcam | ab16666 |
| Alexa 488 anti-rabbit secondary antibody | goat | Thermo Fisher | A-11008 |
| Fibronectin 1 (FN1) | mouse | Thermo Fisher | MA5-1198 |
| Platelet-derived growth factor receptor α (PDGFRα) | rabbit | Cell Signaling Technology | 3174 |
| Vimentin (VIM) | rabbit | Cell Signaling Technology | 5741 |
| α-Smooth muscle actin (αSMA) | rabbit | Abcam | ab5694 |
| Caveolin 1 (CAV1) | rabbit | Cell Signaling Technology | 3267 |
| Fibroblast activating protein (FAP) | rabbit | Cell Signaling Technology | 66562 |
| Podoplanin (PDPN) | rabbit | Cell Signaling Technology | 9047 |
| Thy1 cell surface antigen (THY1) | rabbit | Cell Signaling Technology | 13801 |
Solutions
1. Biopsy collection solution (see Recipes)
2. Digestion solution (see Recipes)
3. PDO isolation wash solution (see Recipes)
4. Fibronectin coating solution (see Recipes)
5. Breast PDO media (see Recipes)
6. Bone metastasis PDO media (see Recipes)
7. Fibroblast media (see Recipes)
8. Freezing media (see Recipes)
9. IF blocking buffer (see Recipes)
10. IF wash buffer (see Recipes)
Recipes
1. Biopsy collection solution (500 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PBS | n/a | 500 mL |
| Pen/Strep 100× | 1× | 5 mL |
| Glucose (200 g/L) | 4.5 g/L | 11.25 mL |
2. Digestion solution (10 mL)
Make fresh for every digestion. Sterile filter before use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Dispase-II solution 1× | n/a | 10 mL |
| Collagenase-I (200 mg/mL) | 2 mg/mL | 100 μL |
| Y-27632 2HCl (5 mM) | 5 μM | 10 μL |
3. PDO wash solution (500 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Advanced DMEM/F12 | n/a | 500 mL |
| Glutamine (200 mM) | 2 mM | 5 mL |
| HEPES (1 M) | 10 mM | 5 mL |
| Pen/Strep (100×) | 1× | 5 mL |
| Primocin (50 mg/mL) | 50 μg/mL | 500 μL |
4. Fibronectin coating solution (5 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PBS | n/a | 5 mL |
| Fibronectin 500 μg/mL | 2 μg/mL | 20 μL |
5. Breast PDO media (50 mL)
Store at 4 °C for a maximum of two weeks. Add Y-27632 2HCl only for the first 4–6 days after isolation.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Advanced DMEM/F12 | n/a | 48 mL |
| Glutamine (200 mM) | 2 mM | 500 μL |
| HEPES (1 M) | 10 mM | 500 μL |
| Pen/Strep (100×) | 1× | 500 μL |
| Primocin (50 mg/mL) | 50 μg/mL | 50 μL |
| B27 supplement (50×) | 1× | 100 μL |
| Nicotinamide (1 M) | 1 mM | 50 μL |
| N-Acetyl-L-Cysteine (500 mM) | 500 μM | 50 μL |
| A82-01 (5 mM) | 500 nM | 5 μL |
| SB202190 (5 mM) | 500 nM | 5 μL |
| 17β-Estradiol (5 μg/mL) | 0.5 ng/mL | 5 μL |
| Hydrocortisone (50 μg/mL) | 50 ng/mL | 50 μL |
| Human EGF (5 μg/mL) | 5 ng/mL | 50 μL |
| Human R-Spondin3 (100 μg/mL) | 200 ng/mL | 100 μL |
| Human noggin (40 μg/mL) | 80 ng/mL | 200 μL |
| Human heregulin-β1 (25 μg/mL) | 25 ng/mL | 50 μL |
| Human FGF7 (10 μg/mL) | 5 ng/mL | 25 μL |
| Human FGF10 (20 μg/mL) | 20 ng/mL | 50 μL |
| Y-27632 2HCl (5 mM) | 5 μM | 10 μL |
6. Bone metastasis PDO media (50 mL)
Store at 4 °C for a maximum of two weeks. Add Y-27632 2HCl only for the first 4–6 days after isolation.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Advanced DMEM/F12 | n/a | 48 mL |
| Glutamine (200 mM) | 2 mM | 500 μL |
| HEPES (1 M) | 10 mM | 500 μL |
| Pen/Strep (100×) | 1× | 500 μL |
| Primocin (50 mg/mL) | 50 μg/mL | 50 μL |
| B27 supplement (50×) | 1× | 100 μL |
| Nicotinamide (1 M) | 1 mM | 50 μL |
| N-Acetyl-L-Cysteine (500 mM) | 500 μM | 50 μL |
| A82-01 (5 mM) | 500 nM | 5 μL |
| SB202190 (5 mM) | 500 nM | 5 μL |
| 17β-Estradiol (5 μg/mL) | 0.5 ng/mL | 50 μL |
| Hydrocortisone (50 μg/mL) | 50 ng/mL | 50 μL |
| Human recombinant EGF (5 μg/mL) | 5 ng/mL | 50 μL |
| Human R-Spondin3 (100 μg/mL) | 200 ng/mL | 100 μL |
| Human noggin (40 μg/mL) | 80 ng/mL | 200 μL |
| Human heregulin-β1 (25 μg/mL) | 25 ng/mL | 50 μL |
| Human FGF7 (10 μg/mL) | 5 ng/mL | 25 μL |
| Human FGF10 (20 μg/mL) | 20 ng/mL | 50 μL |
| Human CXCL12 (10 μg/mL) | 10 ng/mL | 50 μL |
| Human IGF-I (20 μg/mL) | 20 ng/mL | 50 μL |
| Human osteopontin (10 μg/mL) | 10 ng/mL | 50 μL |
| Y-27632 2HCl (5 mM) | 5 μM | 10 μL |
7. Fibroblast media (500 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Human fibroblast expansion basal medium 1× | n/a | 500 mL |
| Low serum growth supplement 50× | 1× | 10 mL |
| FBS | 4% | 10 mL |
| Pen/Strep 100× | 1× | 5 mL |
| Primocin 50 mg/mL | 50 μg/mL | 500 μL |
8. Freezing media (5 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CryoStor CS10 | n/a | 5 mL |
| Y-27632 2HCl (5 mM) | 5 μM | 5 μL |
9. IF blocking buffer (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PBS | n/a | 100 mL |
| BSA | 1% | 1 g |
| Goat serum | 5% | 5 mL |
| Triton X-100 | 0.3% | 300 μL |
10. IF wash buffer (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PBS | n/a | 1,000 mL |
| Triton X-100 | 0.3% | 3 mL |
Laboratory supplies
1. 50 mL centrifuge tube (Celltreat, catalog number: 229106)
2. 15 mL centrifuge tube (Celltreat, catalog number: 229411)
3. 25 mL serological pipettes, individually wrapped (Thermo Fisher, catalog number: 170357N)
4. 10 mL serological pipettes, individually wrapped (Thermo Fisher, catalog number:170356N)
5. 1,000 μL pipette tips (VWR, catalog number: 89082-350)
6. 200 μL pipette tips (VWR, catalog number: 89082-366)
7. 20 μL pipette tips (VWR, catalog number 89082-338)
8. 24-well plates (Thermo Fisher, catalog number: 142475)
9. 6-well plates (Thermo Fisher, catalog number: 140675)
10. 10 cm plates (Thermo Fisher, catalog number: 150350)
11. 1.5 mL microcentrifuge tubes (Eppendorf, catalog number: 0030120086)
12. 1.2 mL cryogenic vials (Corning, catalog number: 430487)
13. Mr. FrostyTM freezing container (Thermo Fisher, catalog number: 5100-0050)
14. TissueTek cryomold (VWR, catalog number: 25608-922)
15. #10 Bard-ParkerTM protected disposable scalpel (Fisher Scientific, catalog number: 02-688-78)
16. #11 Bard-ParkerTM protected disposable scalpel (Fisher Scientific, catalog number: 02-688-79)
17. Tissue cassette (VWR, catalog number: 18000-134)
18. BD disposable syringes (Fisher Scientific, catalog number: 14-823-435)
19. 25 G precision glide needles (Fisher Scientific, catalog number: 14-826-49)
20. FalconTM 4-well chamber slides (Fisher Scientific, catalog number: 08-774-209)
21. Coverglass 24 × 60 (Fisher Scientific, catalog number: NC1672857)
22. Forceps (Fisher Scientific, catalog number: 08-953G)
Equipment
1. Laminar flow hood (Baker, model: SterilGARD)
2. Incubator (Thermo, model: Forma Stericycle i160)
3. Centrifuge (Thermo, model: Sorvall legend XI)
4. Cell counter (Countess II FL)
5. Fisherbrand mini-tube rotator (Thermo Fisher, catalog number: 88-861-051)
6. Thermal mixer (Thermo Fisher, catalog number: 13687712)
7. Microscope (Olympus, model: CK2)
8. Microscope (Olympus, model: BX43)
9. Confocal microscope (Zeiss, model: LSM880)
Software and datasets
1. Zeiss (ZEN lite)
2. Fiji (version 2.14.0/1.54f)
Procedure
文章信息
稿件历史记录
提交日期: Sep 3, 2024
接收日期: Nov 3, 2024
在线发布日期: Dec 5, 2024
出版日期: Jan 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Högström, J. M. and Muranen, T. (2025). An Optimized Protocol for Simultaneous Propagation of Patient-derived Organoids and Matching CAFs. Bio-protocol 15(2): e5160. DOI: 10.21769/BioProtoc.5160.
- Hogstrom, J. M., Cruz, K. A., Selfors, L. M., Ward, M. N., Mehta, T. S., Kanarek, N., Philips, J., Dialani, V., Wulf, G., Collins, L. C., et al. (2023). Simultaneous isolation of hormone receptor–positive breast cancer organoids and fibroblasts reveals stroma-mediated resistance mechanisms. J Biol Chem. 299(8): 105021.
分类
癌症生物学 > 通用技术 > 肿瘤形成 > 细胞分离和培养
细胞生物学 > 细胞分离和培养 > 共培养
干细胞 > 类器官培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link