- EN - English
- CN - 中文
A Highly Efficient System for Separating Glandular and Non-glandular Trichome of Cucumber Fruit for Transcriptomic and Metabolomic Analysis
用于黄瓜果实腺毛与非腺毛分离的高效系统,适用于转录组和代谢组分析
(*contributed equally to this work) 发布: 2025年01月05日第15卷第1期 DOI: 10.21769/BioProtoc.5154 浏览次数: 1466
评审: Wenrong He Kailiang BoAnonymous reviewer(s)
Abstract
Cucumber (Cucumis sativus) trichomes play a critical role in resisting external biological and abiotic stresses. Glandular trichomes are particularly significant as they serve as sites for the synthesis and secretion of secondary metabolites, while non-glandular trichomes are pivotal for determining the appearance quality of cucumbers. However, current methods for separating trichomes encounter challenges such as low efficiency and insufficient accuracy, limiting their applicability in multi-omics sequencing studies. This protocol introduces an efficient system designed for the precise separation of glandular and non-glandular trichomes from cucumber fruit. The process begins with the pre-cooling of sorbitol buffer or ethanol solution and the RNA-free treatment of laboratory supplies, followed by sterilization and pre-cooling. After filling glass bottles with pre-cooling buffer and glass beads, cucumber ovaries are then placed in the glass bottles and the trichome is harvested by bead-beating method. The separation process involves sequential filtration through various steel sieves and centrifugation to separate trichomes. The separated trichomes obtained from this method are well-suited for subsequent multi-omics sequencing analyses. This protocol achieved high precision in separating glandular and non-glandular trichomes, significantly enhancing the efficiency of separation and sample collection processes. This advancement not only addresses existing limitations but also facilitates comprehensive studies aimed at exploring the genetic and biochemical diversity present within cucumber trichomes, thereby opening avenues for broader agricultural and biological research applications.
Key features
• Use cucumber fruits on the day of flowering.
• Pre-cooling and RNA-free treatment ensure supply quality and purity.
• Efficiently separate glandular and non-glandular trichomes.
• Trichome samples are suitable for multi-omics sequencing analysis.
Keywords: Cucumber fruit (黄瓜果实)Graphical overview
Background
With the advancement of biotechnology, there has been a dramatic increase in the number of methods used to separate trichomes in order to study their development, biosynthesis, and metabolism within glandular trichomes. Over the years, methods of separating and collecting trichomes have made great progress in the research process [1].
Earlier methods, such as direct tweezing of trichome cells in tobacco (Nicotiana tabacum) [2] and tweezing of individual trichome cells in Arabidopsis thaliana after quick-freezing plant materials with liquid nitrogen [3] proved inefficient and inaccurate. In tomatoes (Solanum lycopersicum), Pasteur pipettes were employed to collect type VI glandular trichomes from stems and leaves [4]. Advances continued with innovations in mint (Mentha spp.), where chemical and physical methods, aided by buffer solutions, enhanced both plant material protection and glandular trichome yield [5]. These techniques have since been adapted and widely adopted across various plant species [6].
Recent years have seen the bead-beating method emerge as a predominant approach for tomato glandular trichome separation in buffered solutions, followed by filtration and density gradient centrifugation to distinguish between different glandular trichome types [7]. Similarly, this method has proven effective for enriching and purifying cannabis (Cannabis sativa) glandular trichomes [8]. Meanwhile, laser microdissection enables the direct separation of individual glandular trichomes, although its use is time-consuming, inefficient, expensive to collect large numbers of trichomes, and not suitable for large sampling requirements [9].
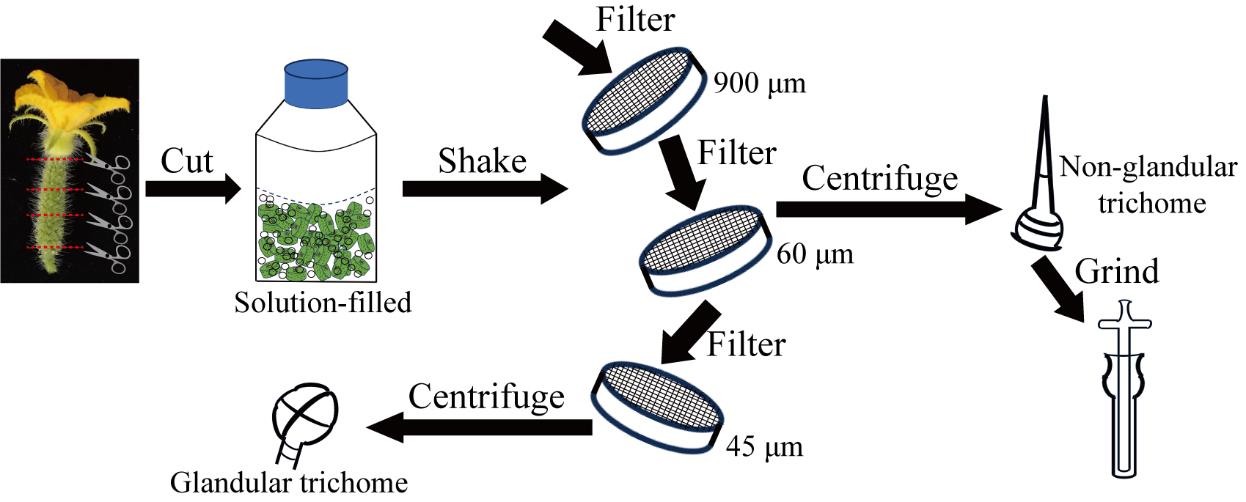
Previous methods, while foundational, have often suffered from limitations in sampling accuracy and efficiency, primarily focusing on glandular trichome separation while neglecting non-glandular trichomes. In addressing these limitations, this protocol focuses on utilizing North China type cucumber fruits on the day of flowering as the primary research materials. Emphasis is placed on optimizing trichome collection and separation methods to ensure adequate sampling and precise separation, encompassing both glandular and non-glandular trichomes. A substantial yield of trichomes was obtained using the bead-beating method, followed by successful separation of glandular and non-glandular trichomes using steel sieves of varying pore sizes. Non-glandular trichome contents were fully extracted via tissue grinding. Standard samples were prepared for subsequent multi-omics sequencing.
This protocol significantly enhances trichome separation efficiency, achieves precise glandular and non-glandular trichome separation, and serves as a valuable reference for trichome separation in other crops and tissues with a certain firmness. This protocol not only refines current methodologies but also underscores the critical role of precise trichome separation in advancing our understanding of plant biology and biotechnology applications.
Materials and reagents
Biological materials
1. North China type cucumber fruits on the day of flowering (Figure 1)

Figure 1. North China type cucumber fruit on the day of flowering
Reagents
1. Sorbitol (Sigma-Aldrich, CAS number: 50-70-4)
2. Tris-HCl (Sigma-Aldrich, CAS number: 1185-53-1)
3. Sucrose (Sigma-Aldrich, CAS number: 57-50-1)
4. KCl (Sigma-Aldrich, CAS number: 7447-40-7)
5. MgCl2 (Sigma-Aldrich, CAS number: 7786-30-3)
6. Succinic acid (Sigma-Aldrich, CAS number: 110-15-6)
7. EGTA (Sigma-Aldrich, CAS number: 67-42-5)
8. K2HPO4 (Sigma-Aldrich, CAS number: 7758-11-4)
9. Triton X-100 (Sigma-Aldrich, CAS number: 9036-19-5)
10. Anhydrous ethanol (Sigma-Aldrich, CAS number: 64-17-5)
11. RNase remover I (Huayueyang Biotechnology, catalog number: 0416-100)
Solutions
1. Sorbitol buffer (see Recipes)
2. 70% (v/v) ethanol (see Recipes)
Recipes
1. Sorbitol buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sorbitol | 200 mM | 18.217 g |
| Tris-HCl | 50 mM | 25 mL |
| Sucrose | 20 mM | 3.4229 g |
| KCl | 10 mM | 0.3725 g |
| MgCl2 | 5 mM | 0.5083 g |
| Succinic acid | 5 mM | 0.2958 g |
| EGTA | 1 mM | 0.1902 g |
| K2HPO4 | 0.5 mM | 0.0435 g |
| Triton X-100 | 0.015% (v/v) | 0.075 mL |
| H2O | n/a | Up to 500 mL |
| Total | n/a | 500 mL |
2. 70% (v/v) ethanol
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Anhydrous ethanol | 70% (v/v) | 350 mL |
| H2O (RNA-free) | n/a | 150 mL |
| Total | n/a | 500 mL |
Laboratory supplies
1. Reagent bottles (Fisher Scientific, catalog number: FB800500)
2. Glass beads (Sangon Biotech, catalog number: A500478)
3. 900 μm steel sieves (Shaoxing Shangyu Shengchao Instrument Equipment, catalog number: 20 Mu)
4. 60 μm steel sieves (Shaoxing Shangyu Shengchao Instrument Equipment, catalog number: 250 Mu)
5. 45 μm steel sieves (Shaoxing Shangyu Shengchao Instrument Equipment, catalog number: 325 Mu)
6. Tissue grinder (Sangon Biotech, catalog number: F519062)
7. 50 mL centrifuge tubes (Sangon Biotech, catalog number: F607788)
8. Petri dishes (Sigma-Aldrich, catalog number: BR455751)
Equipment
1. High-speed refrigerated centrifuge (Bioridge, model: TGL-18M)
2. Optical microscope (OLYMPUS, model: CX23)
Procedure
文章信息
稿件历史记录
提交日期: Jul 17, 2024
接收日期: Nov 3, 2024
在线发布日期: Nov 20, 2024
出版日期: Jan 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Sun, L., Feng, Z., Wang, F., Qi, Y., An, M., Yang, L., Feng, M., Wang, M., Ren, H. and Liu, X. (2025). A Highly Efficient System for Separating Glandular and Non-glandular Trichome of Cucumber Fruit for Transcriptomic and Metabolomic Analysis. Bio-protocol 15(1): e5154. DOI: 10.21769/BioProtoc.5154.
- Feng, Z., Sun, L., Dong, M., Fan, S., Shi, K., Qu, Y., Zhu, L., Shi, J., Wang, W. and Liu, Y. (2023). Novel players in organogenesis and flavonoid biosynthesis in cucumber glandular trichomes. Plant Physiol. 192(4): 2723–2736.
分类
植物科学 > 植物生物化学 > RNA > RNA 提取
分子生物学 > RNA > RNA 提取
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link