- EN - English
- CN - 中文
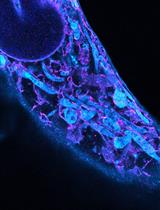
Using HBmito Crimson to Observe Mitochondrial Cristae Through STED Microscopy
利用HBmito Crimson结合STED显微镜观察线粒体嵴结构
(*contributed equally to this work) 发布: 2025年01月05日第15卷第1期 DOI: 10.21769/BioProtoc.5150 浏览次数: 1639
评审: Domenico Azarnia TehranAftab NadeemRozemarijn Van Der Veen

相关实验方案
相关常规和超分辨率光激活定位显微镜 (PALM) 成像来表征活哺乳动物细胞的染色质结构和动力学
Dushyant Mehra and Elias M. Puchner
2023年10月20日 2512 阅读
Abstract
Mitochondrial cristae, formed by folding the mitochondrial inner membrane (IM), are essential for cellular energy supply. However, the observation of the IM is challenging due to the limitations in spatiotemporal resolution offered by conventional microscopy and the absence of suitable in vitro probes specifically targeting the IM. Here, we describe a detailed imaging protocol for the mitochondrial inner membrane using the Si-rhodamine dye HBmito Crimson, which has excellent photophysical properties, to label live cells for imaging via stimulated emission depletion (STED) microscopy. This allows for STED imaging over more than 500 frames (approximately one hour), with a spatial resolution of 40 nm, enabling the observation of cristae dynamics during various mitochondrial processes. The protocol includes detailed steps for cell staining, image acquisition, image processing, and resolution analysis. Utilizing the superior resolution of STED microscopy, the structure and complex dynamic changes of cristae can be visualized.
Key features
• The protocol is designed to visualize mitochondrial cristae in living cells using STED microscopy.
• The protocol enables nanoscale observation of dynamic mitochondrial cristae.
• Real-time observation of mitochondrial morphological changes, fusion, and fission events.
Keywords: Live cell imaging (活细胞成像)Graphical overview
Background
Mitochondria are crucial organelles responsible for ATP production, metabolic regulation, calcium homeostasis, and participation in cellular signaling processes [1–5]. The mitochondrial inner membrane (IM) is a crucial structure within mitochondria, containing the oxidative phosphorylation complexes responsible for ATP synthesis. These complexes on the IM play a key role in cellular energy supply by hosting the entire respiratory chain [6,7]. The IM folds into cristae to increase the surface area, thereby supporting greater ATP production. The shape of the cristae is altered by mitochondrial dynamics, which can affect mitochondrial respiration [8].
The diameter of mitochondria ranges from 200 to 700 nm, with cristae spacing typically around 70 nm [9]. Conventional confocal microscopy has a resolution limited to approximately 200 nm, which is insufficient to resolve the details of these structures; however, the advent of super-resolution microscopy has enabled the visualization of cristae [10]. Stimulated emission depletion (STED) microscopy, in particular, achieves high resolution by employing a doughnut-shaped depletion laser to selectively erase peripheral fluorescence following sample excitation, thereby improving the point spread function (PSF). STED technique provides a spatial resolution of 50 nm and temporal resolution of 1 frame per second, making it a powerful tool for dynamic imaging of mitochondrial cristae at the single-crista level.
In the STED system, the intensity of the depletion beam is several thousand times higher than that of the excitation beam, requiring fluorophores in the fluorescent probes to possess exceptional photostability to avoid quenching. Therefore, we have designed a novel fluorescent dye for STED imaging. HBmito Crimson is characterized by exceptional photostability, targeted accumulation on the mitochondrial IM, and emission only upon binding to the IM, as shown in Figure 1A. In pure organic solvents or water, HBmito Crimson exhibited its absorption peak at 660 nm and an emission peak at 688 nm (Figure 1B). The emission spectrum tail of HBmito Crimson extends toward 775 nm, generating a small peak near this wavelength. This reduces the dye’s saturation power, significantly enhancing the depletion efficiency of the 775 nm laser [11].
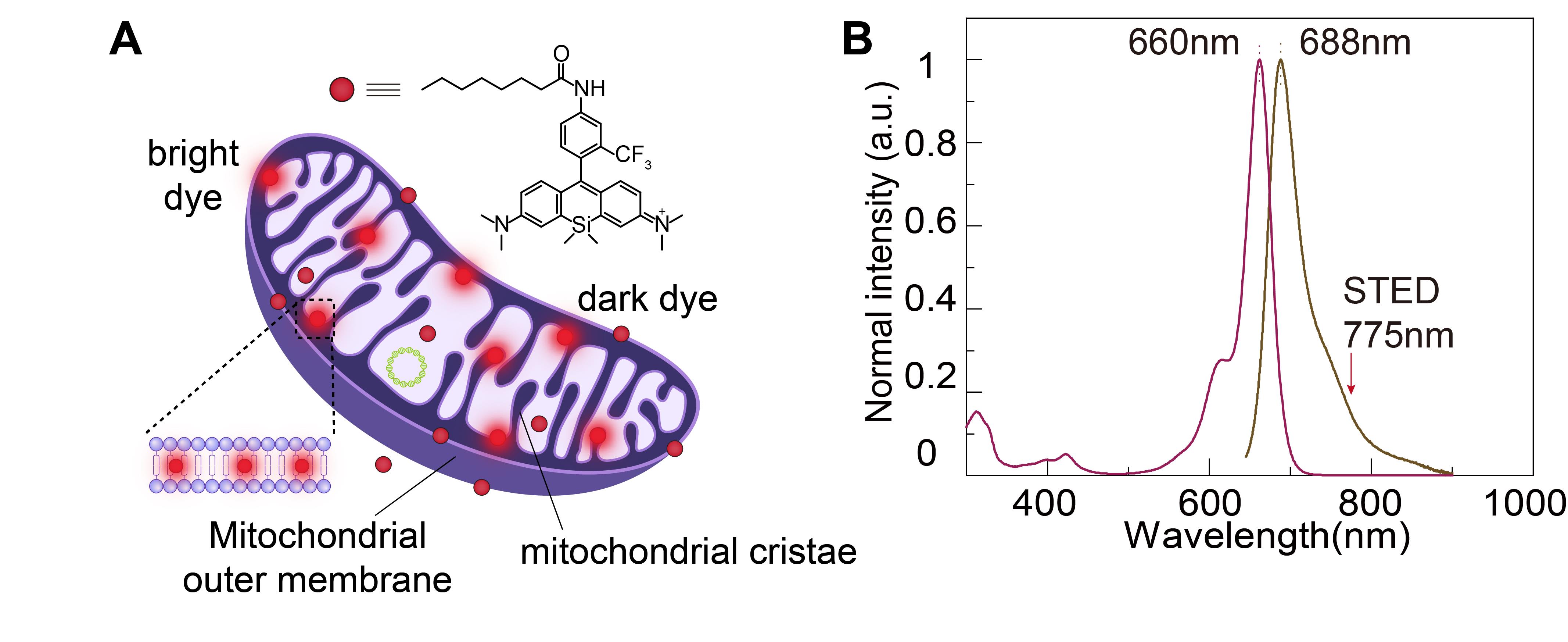
Figure 1. Structural and spectral properties of HBmito Crimson for mitochondrial labeling. A. The chemical structure of HBmito Crimson is used for the specific labeling of the mitochondrial inner membrane. B. The absorption and emission spectra of HBmito Crimson, which can be depleted using a 775 nm laser.
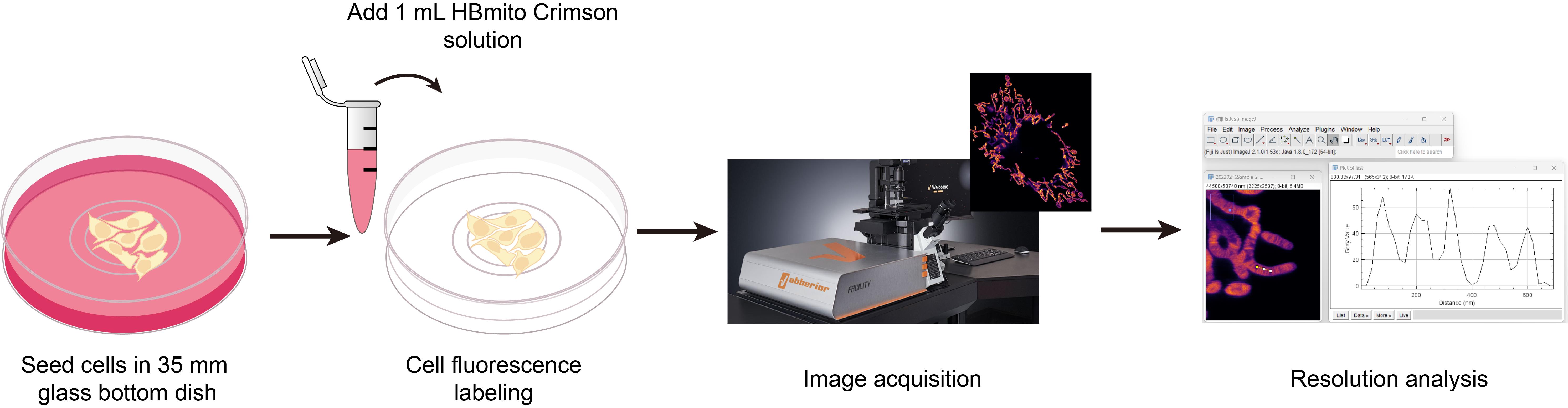
In this protocol, we describe in detail the preparation of cells, labeling methods with HBmito Crimson, image acquisition, and image processing methods suitable for confocal and STED microscopy imaging.
Materials and reagents
Biological materials
1. COS7 cell (ATCC, CRL-1651)
Reagents
1. Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, catalog number: 11965-092), store at 4 °C
2. Fetal bovine serum (FBS) (Gibco, catalog number: 10091148), store at -20 °C
3. Penicillin/streptomycin solution (Gibco, catalog number: 15140122), store at -20 °C
4. Trypsin-EDTA solution (Gibco, catalog number: 25200056), store at -20 °C
5. Dulbecco’s phosphate buffered saline (PBS) (Gibco, catalog number: 11965-092), store at 4 °C
6. HBmito Crimson, 200 μM DMSO solution, store at -20 °C
The dyes in this article can be synthesized and purified according to the literature [11] or purchased from MedChemExpress (catalog number: HY-D2346).
7. DMSO (analytical reagent grade) (Aladdin, catalog number: D103272), store at room temperature
8. Immersion oil (Olympus, catalog number: IMMOIL-F30CC)
Solutions
1. Complete COS7 cell media (see Recipes)
2. HBmito Crimson solution (see Recipes)
3. HBmito Crimson DMSO solution (see Recipes)
Recipes
1. Complete COS7 cell media
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM | n/a | 44.5 mL |
| FBS | 10% | 5 mL |
| Penicillin/streptomycin solution | 1% | 0.5 mL |
| Total | n/a | 50 mL |
The solution should be stored at 4 °C and used within one month. Ensure that it is sterile each time it is used. FBS should be filtered through a 0.2 μm filter membrane.
2. HBmito Crimson solution
| Reagent | Final concentration | Amount |
| HBmito Crimson, 200 μM | 500 nM | 2 μL |
| COS7 cell media | n/a | Up to 1 mL |
| Total | n/a | 1 mL |
Prepare the solution before each use.
3. HBmito Crimson DMSO solution
| Reagent | Final concentration | Amount |
| HBmito Crimson, 5 mM | 5 μM | 1 μL |
| DMSO | n/a | Up to 1 mL |
| Total | n/a | 1 mL |
Prepare the solution before each use.
Laboratory supplies
1. Sterile 1.5 mL polypropylene centrifuge tubes (any vendor)
2. Sterile 50 mL polypropylene centrifuge tubes (any vendor)
3. Sterile nuclease-free filter tips (10, 200, and 1000 μL) (any vendor)
4. 35 mm glass bottom dishes, 170 μm ± 5 μm (Standard Imaging, catalog number: STGBD-035-1; Cellvis, catalog number: C35-20-1.5H)
5. Pipette tips, sterile (any vendor)
6. Cell culture flasks, vented flask surface area 25 cm2 (any vendor)
7. Pipettor (Eppendorf Research® plus) with volume ranges of 0.5–10 μL, 10–100 μL and 100–1000 μL
8. 0.2 μm filter membrane (Millex-GP, catalog number: SLGPR33RB)
Equipment
1. Abberior Facility Line (Abberior Instruments GmbH, Germany) with a 60× oil immersion objective (N.A. 1.42, Olympus, Japan)
2. Common lab equipment: cell culture incubator, safety cabinet, -20°C freezer, fridge (any vendor)
3. Microscope incubator (OKOlab, model: H301-T-UNIT-BL-PLUS)
Software and datasets
1. Abberior Imspector (16.3.14280)
2. Huygens SVI (23.10)
3. ImageJ Fiji, version 2.1.0. with Java 1.8.0_172
4. Origin (2019b)
Procedure
文章信息
稿件历史记录
提交日期: Aug 11, 2024
接收日期: Oct 27, 2024
在线发布日期: Nov 27, 2024
出版日期: Jan 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Ge, X., Ren, W., Shan, C., Xi, P. and Gao, B. (2025). Using HBmito Crimson to Observe Mitochondrial Cristae Through STED Microscopy. Bio-protocol 15(1): e5150. DOI: 10.21769/BioProtoc.5150.
分类
生物物理学 >
细胞生物学 > 细胞成像 > 超分辨率成像
细胞生物学 > 细胞染色 > 细胞器
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link