- EN - English
- CN - 中文
Capacitance Measurements of Exocytosis From AII Amacrine Cells in Retinal Slices
视网膜切片中AII无长突细胞胞吐的电容测量
发布: 2025年01月05日第15卷第1期 DOI: 10.21769/BioProtoc.5147 浏览次数: 2314
评审: Wallace B. ThoresonRuth HeidelbergerAkira KarasawaAnonymous reviewer(s)
Abstract
During neuronal synaptic transmission, the exocytotic release of neurotransmitters from synaptic vesicles in the presynaptic neuron evokes a change in conductance for one or more types of ligand-gated ion channels in the postsynaptic neuron. The standard method of investigation uses electrophysiological recordings of the postsynaptic response. However, electrophysiological recordings can directly quantify the presynaptic release of neurotransmitters with high temporal resolution by measuring the membrane capacitance before and after exocytosis, as fusion of the membrane of presynaptic vesicles with the plasma membrane increases the total capacitance. While the standard technique for capacitance measurement assumes that the presynaptic cell is unbranched and can be represented as a simple resistance-capacitance (RC) circuit, neuronal exocytosis typically occurs at a distance from the soma. Even in such cases, however, it can be possible to detect a depolarization-evoked increase in capacitance. Here, we provide a detailed, step-by-step protocol that describes how "Sine + DC" (direct current) capacitance measurements can quantify the exocytotic release of neurotransmitters from AII amacrine cells in rat retinal slices. The AII is an important inhibitory interneuron of the mammalian retina that plays an important role in integrating rod and cone pathway signals. AII amacrines release glycine from their presynaptic dendrites, and capacitance measurements have been important for understanding the release properties of these dendrites. When the goal is to directly quantify the presynaptic release, there is currently no other competing method available. This protocol includes procedures for measuring depolarization-evoked exocytosis, using both standard square-wave pulses, arbitrary stimulus waveforms, and synaptic input.
Key features
• Quantification of exocytosis with the Sine + DC technique for visually targeted AII amacrines in retinal slices, using voltage-clamp and whole-cell patch-clamp recording.
• Because exocytosis occurs away from the somatic recording electrode, the sine wave frequency must be lower than for the standard Sine + DC technique.
• Because AII amacrines are electrically coupled, the sine wave frequency must be sufficiently high to avoid interference from other cells in the electrically coupled network.
• The protocol includes procedures for measuring depolarization-evoked exocytosis using standard square-wave pulses, stimulation with arbitrary and prerecorded stimulus waveforms, and activation of synaptic inputs.
Keywords: AII amacrine cell (AII无长突细胞)Graphical overview
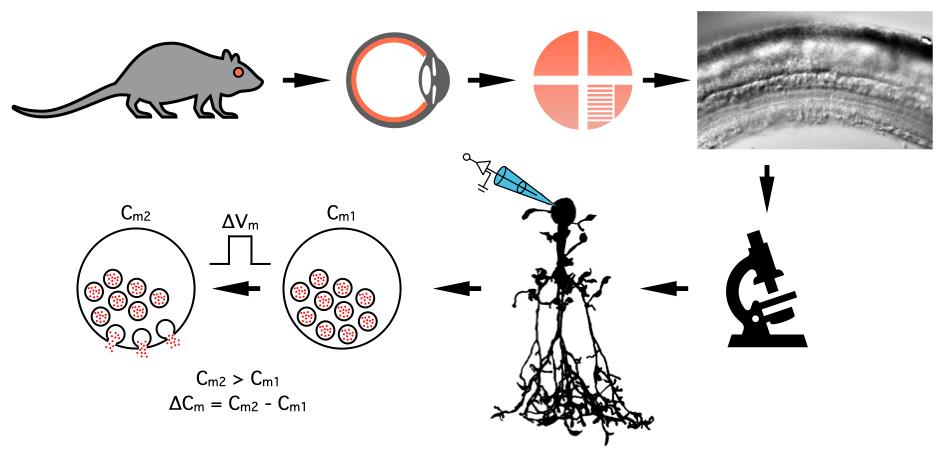
Measuring changes in the membrane capacitance of AII amacrine cells during whole-cell patch-clamp recording in rat retinal slices
Background
In a chemical synapse, a neurotransmitter is released by exocytosis from the presynaptic neuron [1]. For a morphologically discrete synapse, the neurotransmitter diffuses across the synaptic cleft, binds to postsynaptic, ligand-gated ion channels, and typically increases their open probability. This can be measured electrophysiologically as a postsynaptic change in current (voltage clamp) or change in voltage (current clamp). Under ideal conditions, the evoked current will directly represent the underlying conductance change but will only be indirectly related to the magnitude and time course of the presynaptic exocytosis. Because the exocytosis corresponds to the fusion of synaptic vesicles with the presynaptic plasma membrane, the presynaptic capacitance will increase in proportion to the summed capacitance of all released vesicles. The capacitance can be measured with high temporal resolution using a lock-in amplifier, i.e., a phase-sensitive detector, implemented in hardware or software.
Standard capacitance measurement of exocytosis assumes an unbranched cell, represented by a simple RC circuit [2,3]. With the branched morphology of neurons, it is of interest to extend capacitance measurements to such structures [4,5]. Over the last 30 years or so, whole-cell recordings for measuring capacitance have been made directly at different presynaptic boutons where exocytosis takes place, e.g., mossy fiber boutons in the hippocampus [6], goldfish bipolar cell terminals [7], rat rod bipolar cell terminals [8], calyx of Held terminals [9,10], and posterior pituitary gland terminals [11].
Attempts have also been made to measure exocytosis occurring at a distance from the recording pipette, e.g., using somatic recordings of mouse rod bipolar cells with very short axons [12]. More recently, capacitance measurements were extended to AII amacrine cells in mouse retina [13]. The AII is an axonless retinal interneuron with presynaptic dendrites that provide glycinergic synapses onto OFF-cone bipolar cells and OFF-ganglion cells [14]. Using activation of voltage-gated Ca2+ channels to trigger exocytosis, Balakrishnan et al. [13] used capacitance measurements to characterize several important functional properties of the glycinergic synapses. It is a problem for the interpretation of their results, however, that exocytosis in AIIs takes place at a distance from the soma and that these cells are electrically coupled (via gap junctions) to each other and to ON-cone bipolar cells [15,16]. If the goal is to measure the true capacitance increase following exocytosis distributed across several presynaptic dendrites, several constraints apply. First, because exocytosis occurs at a distance from the somatic pipette, the sine wave frequency used to measure the capacitance must be low enough that the electrotonic attenuation from the soma does not exclude some presynaptic terminals from contributing to the measurements. On the other hand, the sine wave frequency must be high enough that attenuation prevents electrotonic transmission through gap junctions that couple to neighboring cells, which can compromise the measurements. Using recently developed compartmental models of AII amacrine cells [17], it was possible to explore these issues computationally and estimate a range of sine wave frequencies that optimizes the trade-off between these conflicting demands [18].
Materials and reagents
Biological materials
1. Rat (Wistar HanTac, Taconic Bioscience)
Reagents
1. Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: 71376 1 kg, CAS number: 7647-14-5)
2. Sodium hydrogen carbonate (NaHCO3) (Sigma-Aldrich, catalog number: S6014 500 g, CAS number: 144-55-8)
3. Potassium chloride (KCl) (Sigma-Aldrich, catalog number: 60128 250 g, CAS number: 7447-40-7)
4. Calcium chloride dihydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: 21097 250 g, CAS number: 10035-04-8)
5. Magnesium chloride hexahydrate (MgCl2·6H2O) (Sigma-Aldrich, catalog number: 63064 500 g, CAS number: 7791-18-6)
6. D-Glucose (Sigma-Aldrich, catalog number: G-8280 1 kg, CAS number: 50-99-7)
7. Potassium gluconate (K-gluconate) (Sigma-Aldrich, catalog number G4500 100 g, CAS number: 299-27-4)
8. Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: 60369 500 g, CAS number: 1310-58-3)
9. Cesium methanesulfonate (CsCH3SO3) (Sigma-Aldrich, catalog number: 368903 25 g, CAS number: 2550-61-0)
10. Cesium chloride (CsCl) (Sigma-Aldrich, catalog number: 1020390050, CAS number: 7647-17-8)
11. Cesium hydroxide (CsOH), 50% (wt) solution in H2O (Sigma-Aldrich, catalog number: 232068 100 g, CAS number: 21351-79-1)
12. Tetraethylammonium chloride (TEA-Cl, (C2H5)4NCl) (Sigma-Aldrich, catalog number: T2265 100 g, CAS number: 56-34-8)
13. 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) (Sigma-Aldrich, catalog number: H3375 100 g, CAS number: 7365-45-9)
14. HEPES, hemisodium salt (hemi-Na salt) (Sigma-Aldrich, catalog number: H7637 100 g, CAS number: 103404-87-1)
15. Ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid (EGTA) (Fluka, catalog number: 03778 50 g, CAS number: 67-42-5)
16. Adenosine 5'-triphosphate magnesium salt (MgATP) (magnesium ATP) (Sigma-Aldrich, catalog number: A9187 1 g, CAS number: 74804-12-9)
17. Guanosine 5'-triphosphate sodium salt (Na3GTP) (sodium GTP) (Sigma-Aldrich, catalog number: G8877 100 mg, CAS number: 36051-31-7)
18. Alexa Fluor 594 hydrazide, Na salt (Thermo Fisher Scientific, Invitrogen, catalog number: A10438)
19. Alexa Fluor 488 hydrazide, Na salt (Thermo Fisher Scientific, Invitrogen, catalog number: A10436)
20. (-)-Bicuculline methochloride (HelloBio, catalog number: HB0895 50 mg, CAS number: 38641-83-7)
21. Strychnine hydrochloride (Research Biochemicals Int., catalog number: S-124). For a current source, see Sigma-Aldrich, catalog number: S8753 (25 g, CAS number: 1421-86-9)
22. 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX) (HelloBio, catalog number: HB0205 10 mg, CAS number: 479345-85-8)
23. (RS)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP) (HelloBio, catalog number: HB0036 50 mg, CAS number: 100828-16-8)
24. Tetrodotoxin, citrate salt (TTX) (HelloBio, catalog number: HB1035 1 mg, CAS number: 18660-81-6)
25. Ames medium powder [Sigma-Aldrich, catalog number: A1420 (10X1L)]
26. N-(2,6-Dimethylphenylcarbamoylmethyl)triethylammonium chloride (QX314 chloride) (Tocris, catalog number: 2313, CAS number: 5369-03-9)
27. Isoflurane for gas anesthesia (Zoetis Animal Health ApS, catalog number: 002185)
28. Acetone for cleaning platinum-iridium wire before gluing nylon strings onto it to make a slice harp (Merck, catalog number: 1.00014, CAS number: 67-64-1)
29. Sodium hypochlorite (NaOCl) (4% in water, chlorine bleach; can be obtained from the local grocery store)
Solutions
1. Extracellular buffer solution used for dissection (EC3000) (see Recipes)
2. Extracellular bath solution (EC1000) (see Recipes)
3. Intracellular pipette stock solution at 1.25× concentration (IC8503) (see Recipes)
4. Intracellular pipette solution at 1× concentration (IC8503) (see Recipes)
5. Intracellular pipette stock solution at 1.25× concentration (IC4101) (see Recipes)
6. Intracellular pipette solution at 1× concentration (IC4101) (see Recipes)
7. Intracellular pipette stock solution at 1.25× concentration (IC4202) (see Recipes)
8. Intracellular pipette solution at 1× concentration (IC4202) (see Recipes)
9. QX314 (stock solution, 50 mM) (see Recipes)
10. Alexa 594 (stock solution, 1 mM) (see Recipes)
11. Alexa 488 (stock solution, 1 mM) (see Recipes)
12. KCl (stock solution, 1 M) (see Recipes)
13. MgCl2 (stock solution, 1 M) (see Recipes)
14. CaCl2 (stock solution, 1 M) (see Recipes)
15. KOH (to adjust pH, 2 M) (see Recipes)
16. KOH (to adjust pH, 0.2 M) (see Recipes)
17. Ames stock solution (see Recipes)
18. Ames storage (incubation) solution (see Recipes)
19. CNQX (stock solution, 100 mM) (see Recipes)
20. Bicuculline (stock solution, 10 mM) (see Recipes)
21. Strychnine (stock solution, 10 mM) (see Recipes)
22. CPP (stock solution, 50 mM) (see Recipes)
23. TTX (stock solution, 0.3 mM) (see Recipes)
Note: Here and later, the numbers used to identify specific extra- and intracellular solutions are essentially arbitrary and follow a system used in our laboratory (based on the functionality of the Patchmaster software from HEKA Elektronik).
Recipes
1. Extracellular buffer solution used for dissection (EC3000)
| Reagent | Final concentration | Quantity or Volume (for 1 L) |
|---|---|---|
| NaCl | 145 mM | 8.474 g |
| HEPES (hemi-Na salt) | 5 mM | 1.247 g |
| KCl | 2.5 mM | 2.5 mL of 1 M stock |
| CaCl2 | 2.5 mM | 2.5 mL of 1 M stock |
| MgCl2 | 1 mM | 1 mL of 1 M stock |
| D-Glucose | 10 mM | 1.802 g |
| H2O (MilliQ) | n/a | to 1,000 mL |
| Total | n/a | 1,000 mL |
Adjust to pH 7.4 with 1 M HCl. Prepare 1,000 mL each time and store at 4 °C. Typically used within a week, keep for up to 10 days.
2. Extracellular bath solution (EC1000)
| Reagent | Final concentration | Quantity or Volume (for 2 L) |
|---|---|---|
| NaCl | 125 mM | 14.610 g |
| NaHCO3 | 25 mM | 4.2 g |
| KCl | 2.5 mM | 5 mL of 1 M stock |
| CaCl2 | 2.5 mM | 5 mL of 1 M stock |
| MgCl2 | 1 mM | 2 mL of 1 M stock |
| D-Glucose | 10 mM | 3.604 g |
| H2O (MilliQ) | n/a | to 2,000 mL |
| Total | n/a | 2,000 mL |
Prepare 2,000 mL for each experiment. Add all ingredients except CaCl2 to a 2 L volumetric flask. Fill up with H2O but leave enough space for the addition of 5 mL 1 M CaCl2. After all solids have been dissolved and the solution is well mixed, pour into a glass bottle that will be used for the rest of the experiment. Osmolality ~300 mOsm.
Note: Do not add CaCl2 before the solution has been saturated with CO2 (see section F below). If the solution has not been saturated with CO2, Ca2+ will precipitate as CaCO3.
3. Intracellular pipette stock solution at 1.25× concentration (IC8503)
| Reagent | Final concentration | Quantity or Volume (for 1.25× concentration) |
|---|---|---|
| CsCH3SO3 | 80 mM | 2.280 g (for 100 mL) |
| CsCl | 40 mM | 0.8418 g (for 100 mL) |
| TEA-Cl | 10 mM | 0.2071 g (for 100 mL) |
| HEPES | 28 mM | 0.8358 g (for 100 mL) |
| EGTA | 2 mM | 0.0951 g (for 100 mL) |
| MgATP | 3 mM | 0.07134 g (for 40 mL) |
| Na3GTP | 1 mM | 0.024654 g (for 40 mL) |
| CsOH (adjust pH to 7.3) | n/a | n/a |
| H2O (MilliQ) | n/a | n/a |
Making a stock solution at 1.25× concentration that gets diluted to a final 1× concentration for the experiment provides flexibility with respect to adding fluorescent dye and specific pharmacological agents. Make up a 40 mL stock solution at 1.25× concentration and store 1 mL aliquots at -20 °C. On the day of the experiment (or shortly before), dilute the 1.25× solution to 1× final concentration by adding water before use. If one decides to also add fluorescent dye (dissolved in water) and/or specific pharmacological compounds (dissolved in water), the volume of water is reduced correspondingly such that the final volume is correct for a 1× solution (see example Recipe below).
In the example Recipe described here, first make up 100 mL of solution at 1.25× concentration containing CsCH3SO3, CsCl, TEA-Cl, HEPES, and EGTA and adjust the pH to 7.3 (with CsOH). From this solution, measure out 40 mL, dissolve the calculated amounts of MgATP and Na3GTP and adjust the pH to 7.3 (only a small amount of CsOH is needed for the second adjustment). Store 1 mL aliquots at -20 °C and dilute to 1× before use.
Caution: CsOH is a very strong base and must be handled with care.
Note: When making up an intracellular pipette solution, there are mutual constraints that influence the accuracy of the different concentrations, the stability of specific compounds, and the total cost of the chemical compounds. On the one hand, preparing a larger volume and adding a larger amount of each chemical increases the accuracy of the concentrations. On the other hand, preparing a smaller volume decreases the total cost. The example here attempts to reach a reasonable compromise and involves preparing a larger initial volume with less expensive compounds, from which a smaller volume is used to prepare the final stock solution. When adjusting the pH (and ideally also the osmolality) of the final solution, there are two challenges. First, volumetric flasks used to prepare solutions with accurate final volumes do not lend themselves to measuring pH using conventional pH electrodes. Second, the base (or acid) that needs to be added cannot be too diluted, as this tends to increase the final volume too much, and also cannot be too concentrated, as it becomes difficult to reach the desired pH without overshooting. One way of handling these problems is to reduce the volume of the solution for which pH is adjusted, approximately by the expected volume (ideally a little less) of base (or acid) whereby pH is adjusted. When the pH has been adjusted to the desired value, the final volume can be checked again in a volumetric flask and, if necessary, H2O can be added.
Note: The water content of ATP and GTP salts varies on a batch-by-batch basis. For consistency, it is therefore recommended to calculate the amounts needed for anhydrous compounds and update the calculations according to the exact water content of a given batch.
4. Intracellular pipette solution at 1× concentration (IC8503)
| Reagent | Final concentration | Quantity or Volume (for 500 μL) |
|---|---|---|
| IC8503 at 1.25× | 1× | 400 μL |
| Alexa 594 | 50 μM | 25 μL of 1 mM stock |
| QX314 chloride | 2 mM | 40 μL of 50 mM stock |
| H2O (MilliQ) | n/a | 35 μL |
| Total | n/a | 500 μL |
After making up 500 μL of intracellular solution at 1× concentration, filter the solution using a 0.22 μm Millex syringe filter. Keep the aliquots on ice during the experiment and freeze at -20 °C between experiments.
Note: Most experimental designs will want to block the Nav channels that mediate spiking in AII amacrine cells for capacitance measurements of exocytosis. One possible solution is to add TTX, a selective blocker of (most types of) Nav channels, to the extracellular bath solution. However, TTX is fairly expensive, and another method is to add the Nav channel blocker QX314, a membrane-impermeable derivative of lidocaine, to the intracellular solution. For a neuron like the AII amacrine cell, Nav channels are blocked within a few minutes after establishing the whole-cell configuration, corresponding to the time it takes for diffusion of QX314 to the subcellular location of the Nav channels.
Note: It is recommended to protect fluorescent dyes from light exposure by covering the corresponding vials with aluminum foil and/or keeping them in a light-tight container.
5. Intracellular pipette stock solution at 1.25× concentration (IC4101)
| Reagent | Final concentration | Quantity or Volume (for 100 mL of 1.25× concentration) |
|---|---|---|
| K-gluconate | 125 mM | 3.6594 g (for 100 mL) |
| NaCl | 8 mM | 1 mL of 1 M stock (for 100 mL) |
| CaCl2 | 1 mM | 0.125 mL of 1 M stock (for 100 mL) |
| HEPES | 10 mM | 0.2975 g (for 100 mL) |
| EGTA | 5 mM | 0.2378 g (for 100 mL) |
| MgATP | 3 mM | 0.05925 g (for 20 mL) |
| KOH (adjust pH to 7.3) | n/a | n/a |
| H2O (MilliQ) | n/a | n/a |
Make up 100 mL of solution with K-gluconate, NaCl, CaCl2, HEPES, and EGTA and adjust pH to 7.3 with KOH. From this solution, measure out 20 mL and add MgATP. Adjust pH to 7.3 with KOH. Store 1 mL aliquots at -20 °C. On the day of the experiment (or shortly before), dilute the 1.25× solution to 1× final concentration by adding water before use. If one decides to also add fluorescent dye (dissolved in water) and/or specific pharmacological compounds (dissolved in water), the volume of water is reduced correspondingly such that the final volume is correct for a 1× solution (see example Recipe below).
Caution: KOH is a very strong base and must be handled with care.
6. Intracellular pipette solution at 1× concentration (IC4101)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| IC4101 at 1.25× | 1× | 400 μL |
| Alexa 488 | 100 μM | 50 μL of 1 mM stock |
| QX314 chloride | 2 mM | 40 μL of 50 mM stock |
| H2O (MilliQ) | n/a | 10 μL |
| Total | n/a | 500 μL |
After making up 500 μL of intracellular solution at 1× concentration, filter the solution using a 0.22 μm Millex syringe filter. Keep the aliquots on ice during the experiment and freeze at -20 °C between experiments.
Note: It is recommended to protect fluorescent dyes from light exposure by covering the corresponding vials with aluminum foil and/or keeping them in a light-tight container.
7. Intracellular pipette stock solution at 1.25× concentration (IC4202)
| Reagent | Final concentration | Quantity or Volume (for 100 mL of 1.25× concentration) |
|---|---|---|
| K-gluconate | 125 mM | 3.6594 g (for 100 mL) |
| KCl | 5 mM | 0.625 mL of 1 M stock (for 100 mL) |
| NaCl | 8 mM | 1 mL of 1 M stock (for 100 mL) |
| HEPES | 10 mM | 0.2975 g (for 100 mL) |
| EGTA | 0.2 mM | 0.0951 g (for 100 mL) |
| MgATP | 4 mM | 0.1185 g (for 40 mL) |
| Na3GTP | 1 mM | 0.0123 g (for 40 mL) |
| KOH (adjust pH to 7.3) | n/a | n/a |
| H2O (MilliQ) | n/a | n/a |
Make up 100 mL solution at 1.25× concentration with K-gluconate, KCl, NaCl, HEPES, and EGTA and adjust pH to 7.3 with KOH. From this solution, measure out 40 mL and add MgATP and Na3GTP. Adjust pH to 7.3 with KOH. Store 1 mL aliquots at -20 °C. On the day of the experiment (or shortly before), dilute the 1.25× solution to 1× final concentration by adding water before use. If one decides to also add fluorescent dye (dissolved in water) and/or specific pharmacological compounds (dissolved in water), the volume of water is reduced correspondingly such that the final volume is correct for a 1× solution (see example Recipe below).
Caution: KOH is a very strong base and must be handled with care.
8. Intracellular pipette solution at 1× concentration (IC4202)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| IC4202 at 1.25× | 1× | 400 μL |
| Alexa 594 | 50 μM | 25 μL of 1 mM stock |
| H2O (MilliQ) | n/a | 75 μL |
| Total | n/a | 500 μL |
After making up 500 μL of intracellular solution at 1× concentration, filter the solution using a 0.22 μm Millex syringe filter. Keep the aliquots on ice during the experiment and freeze at -20 °C between experiments.
Note: It is recommended to protect fluorescent dyes from light exposure by covering the corresponding vials with aluminum foil and/or keeping them in a light-tight container.
9. QX314 (stock solution, 50 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| QX314 chloride | 50 mM | 10 mg |
| H2O (MilliQ) | n/a | 0.67 mL |
| Total | n/a | 0.67 mL |
MW 298.85 g/mol. Store at -20 °C in 100 μL aliquots.
10. Alexa 594 (stock solution, 1 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Alexa Fluor 594, hydrazide, Na salt | 1 mM | 1 mg |
| H2O (MilliQ) | n/a | 1.32 mL |
| Total | n/a | 1.32 mL |
MW 758.79 g/mol. Store at -20 °C in 50 μL aliquots.
11. Alexa 488 (stock solution, 1 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Alexa Fluor 488, hydrazide, Na salt | 1 mM | 1 mg |
| H2O (MilliQ) | n/a | 1.75 mL |
| Total | n/a | 1.75 mL |
MW 570.48 g/mol. Store at -20 °C in 50 μL aliquots.
12. KCl (stock solution, 1 M)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| KCl | 1 M | 7.456 g |
| H2O (MilliQ) | n/a | to 100 mL |
| Total | n/a | 100 mL |
MW 74.55 g/mol. Prepare 100 mL each time, using a 100 mL volumetric flask. Store at room temperature, preferably in the dark. Keep for up to 4 weeks.
13. MgCl2 (stock solution, 1 M)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| MgCl2·6H2O | 1 M | 10.166 g |
| H2O (MilliQ) | n/a | to 50 mL |
| Total | n/a | 50 mL |
MW 203.30 g/mol. Prepare 50 mL each time, using a 50 mL volumetric flask. Store at room temperature, preferably in the dark. Keep for up to 4 weeks.
Note: Please note that MgCl2 is very hygroscopic and will absorb water. Depending on the extent to which this happens, the true amount of salt added will be reduced. To prevent (or minimize) this problem, only purchase relatively small amounts that will be consumed over a reasonable period of time, keep the container tightly closed, and only open the container briefly when weighing out material.
14. CaCl2 (stock solution, 1 M)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CaCl2·2H2O | 1 M | 14.701 g |
| H2O (MilliQ) | n/a | to 100 mL |
| Total | n/a | 100 mL |
MW 147.01 g/mol. Prepare 100 mL each time, using a 100 mL volumetric flask. Store at room temperature, preferably in the dark. Keep for up to 4 weeks.
Note: Please note that CaCl2 is very hygroscopic and will absorb water. See note for recipe 13 above.
15. KOH (to adjust pH, 2 M)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| KOH | ~2 M | ~100 mg |
| H2O (MilliQ) | n/a | 1 mL |
| Total | n/a | 1 mL |
MW 56.11 g/mol.
Caution: KOH is a very strong base and must be handled with care. Because of potential ion exchange, it is recommended to prepare solutions of KOH in plastic containers (not glassware).
Note: For adjusting pH in intracellular pipette solutions based on K+ salts. KOH comes in the form of pellets, with one pellet weighing approximately 100 mg. To adjust pH, it is useful to have a solution of KOH at approximately 2 M, corresponding to one pellet dissolved in 1 mL of H2O. In addition to the 2 M KOH solution, it is useful to also have a 0.2 M solution of KOH; see recipe below. When adjusting the pH of s small volume of intracellular pipette solution, it is useful to start by adding KOH at a high concentration such that the volume of the solution does not change much. When the pH has almost reached the target value, continuing with the high concentration risks overshooting the target value. Instead, add KOH at the lower concentration (0.2 M).
16. KOH (to adjust pH, 0.2 M)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| KOH | ~0.2 M | ~0.1 mL of 2 M stock solution |
| H2O (MilliQ) | n/a | 0.9 mL |
| Total | n/a | 1 mL |
See comments above for 2 M KOH.
17. Ames stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ames medium powder | n/a | 8.8 g (1 glass vial for 1 L) |
| H2O (MilliQ) | to 1,000 mL | |
| Total | n/a | to 1,000 mL |
Prepare 1,000 mL each time and store 50 mL aliquots at -20 °C.
18. Ames storage (incubation) solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ames stock solution | n/a | 50 mL |
| NaHCO3 | 25 mM | 105 mg (for 50 mL) |
| Total | n/a | 50 mL |
Thaw a 50 mL aliquot on the day of the experiment. Bubble solution with a gas composed of 95% O2 and 5% CO2 for approximately 20 min (until solution is saturated with CO2). Then, add 105 mg of NaHCO3 and stir until dissolved. Discard the solution after the experiment day.
Note: If NaHCO3 is added before the solution is saturated with CO2, Ca2+ will precipitate as CaCO3.
19. CNQX (stock solution, 100 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CNQX | 100 mM | 10 mg |
| H2O (MilliQ) | n/a | 362 μL |
| Total | n/a | 362 μL |
MW 276.12 g/mol. Store at -20 °C in 50 μL aliquots.
Caution: CNQX may be toxic and must be handled with care.
20. Bicuculline (stock solution, 10 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Bicuculline methochloride | 10 mM | 50 mg |
| H2O (MilliQ) | n/a | 11.96 mL |
| Total | n/a | 11.96 mL |
MW 417.85 g/mol. Store at -20 °C in 500 μL aliquots.
Caution: Bicuculline is toxic and must be handled with care.
21. Strychnine (stock solution, 10 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Strychnine hydrochloride × 1.75H2O | 10 mM | 201.2 mg |
| H2O (MilliQ) | n/a | to 50 mL |
| Total | n/a | 50 mL |
MW 402.38 g/mol (including 1.75 × H2O). Store at -20 °C in 1 mL aliquots.
Caution: Strychnine is toxic and must be handled with care.
22. CPP (stock solution, 50 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CPP | 50 mM | 50 mg |
| H2O (MilliQ) | n/a | 3.96 mL |
| Total | n/a | 3.96 mL |
MW 252.21 g/mol. Store at -20 °C in 100 μL aliquots.
Caution: CPP may be toxic and must be handled with care.
23. TTX (stock solution, 0.3 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| TTX | 1 mM | 1 mg |
| H2O (MilliQ) | n/a | 10.44 mL |
| Total | n/a | 10.44 mL |
MW 319.27 g/mol. Store at -20 °C in 500 μL aliquots.
Caution: TTX is toxic and must be handled with care.
Laboratory supplies
1. Plastic Petri dish 100 × 15 mm (Corning Inc., catalog number: 351029)
2. Scalpel holder #4 (Fine Science Tools, catalog number: 10004-13)
3. Scalpel blade #20 (Swann Morton Ltd., catalog number: 0086)
4. Scissor, curved, for dissection (B. Braun, catalog number: BC061R)
5. Scissor, small for dissecting eyeball (Fine Science Tools, catalog number: 15000-10)
6. Watchmaker's forceps #5 (VWR, catalog number: 232-1221)
7. Pasteur pipette, with gently fire-polished tip (VWR, catalog number: 612-1709)
8. Borosilicate glass for making patch pipettes (filamented, thick-walled; outer diameter, 1.5 mm; inner diameter, 0.86 mm) (Sutter Instrument, catalog number: BF150-86-10)
9. Parafilm (American National Can, catalog number: 06830)
10. Injection needle, 21 G (Becton, Dickinson and Company, catalog number: 301155)
11. Syringe, 1 mL (Becton, Dickinson and Company, catalog number: 300013)
12. VitraPOR micro-filter-candle tube for bubbling gas in bath solutions, 13 × 25 mm, 8 mm diameter tube, porosity #4 (ROBU Glasfilter-Geraete, catalog number: 18124)
13. Cell strainer, BD Falcon, 100 μm nylon mesh (BD Biosciences, catalog number: 352360)
14. Storage chamber for retinal flatmount pieces (custom-made interface chamber), see section B
15. Plastic box (for making a storage chamber for retinal flatmount pieces, see section B
16. Lens paper (Karl Hecht Assistent, catalog number: 41019010). Cut into small pieces (approximately 15 mm × 5 mm) and store in a small Petri dish
17. Platinum-iridium (Pt-Ir) wire, diameter 0.5 mm, 0.5 mm × 30 cm (World Precision Instruments, catalog number: PTP201)
18. Nylon strings, isolated from nylon stocking
19. Cyanoacrylate (super glue)
20. RTV118 silicone rubber adhesive sealant (Momentive Performance Materials, catalog number: RTV118-85ML)
21. Millex-GV 0.22 μm syringe driven filter tips (Millipore/Merck, catalog number: SLGV004SL)
22. Microloader tips (Eppendorf, catalog number: 5242956.003)
23. Adjustable tubing clamps, "stop-it hose clamp Easy-Click," 10 and 15 mm diameter (Bürkle, catalog number: 8619-0102, 8619-0155)
24. Ag-wire for ground electrodes (patch pipette, bath chamber), Teflon-coated, diameter 0.015" (0.38 mm) (World Precision Instruments, catalog number AGT1510)
25. Small glass beakers, 25 mL (VWR, catalog number: 213-1120)
26. Silicone tubing (thick), ID 5 mm, OD 8 mm (VWR, catalog number: 288-0714)
27. Silicone tubing (thin), ID 2 mm, OD 4 mm (VWR, catalog number: 228-0704P)
28. Tygon tubing (thick), ID 1/16", OD 3/16" (Saint-Gobain Performance Plastics, part number: AAC02002)
29. Tygon tubing (thin), ID 1/16", OD 1/8" (Saint-Gobain Performance Plastics, part number: AAC00002)
Equipment
1. Patch-clamp amplifier (HEKA Elektronik, model: EPC10)
2. Model cell circuit (HEKA Elektronik, model: MC 10)
3. Personal computer for data acquisition and experiment control (Apple Macintosh or Windows PC)
4. Upright, fixed-stage microscope (Olympus/Evident, model BX51WI)
5. Infrared (IR) video camera (TILL Photonics, catalog number: VX55)
6. TV monitor, black/white (CBC Co. Ltd., model CEM-15A)
7. Recording bath chamber insets (aluminum, Teflon-coated) for in vitro slices (Luigs & Neumann, catalog number: 200-100 500 0180-0B), see section C
8. Cover glass (Menzel Gläser), for bottom of recording bath chambers, diameter 50 mm, type #1 (VWR, catalog number: 630-2129), see section C
9. Fluorescence light source for microscope
10. Water immersion objective (×40 or ×60, Olympus/Evident)
11. Dodt gradient contrast (DGC) tube (Luigs & Neumann)
12. Micromanipulator Mini25 motorized (Luigs & Neumann)
13. Fluorescence imaging system (widefield or 2-photon)
14. Vibration isolation table (Technical Manufacturing Corporation [TMC], "Micro-g", model number 63-540)
15. Faraday cage (custom-made)
16. Micro-Osmometer (based on the technique of freezing-point depression to measure osmolality of intracellular pipette solutions) (Fiske Associates, model: 210)
17. Dissection microscope (Leica, model: S6E)
18. Light source for dissection microscope (Volpi, model: Interlux 4100)
19. pH meter (Hanna, catalog number: HI8424)
20. Digital manometer ± 1 psi, incl. custom-made sensor (Sigmann Elektronik, catalog number: 3000703)
21. Water jet pump (BRAND GmbH, catalog number: 1596 00), can be replaced with an electric pump if the use of a water jet pump is not recommended/permitted
22. Pipette puller (Narishige, catalog number: PP-83)
Note: For several items, equivalent commercial alternatives are available. For contrast enhancement, infrared differential interference contrast (IR-DIC) microscopy is an alternative to infrared Dodt gradient contrast (IR-DGC) microscopy.
Software and datasets
1. JPCalcW (Molecular Devices) or JPCalcWin (SDR Scientific), requires license. The Patcher's Power Tools is a free package (required IGOR Pro) that contains some functionality for calculating liquid junction potentials (https://www3.mpibpc.mpg.de/groups/neher/index.php?page=software)
2. Patchmaster v2x92 (HEKA Elektronik/MultiChannel Systems), requires license
3. Fitmaster v2x92 (HEKA Elektronik/MultiChannel Systems), requires license
4. IGOR Pro v9 (WaveMetrics/Sutter Instrument), requires license
Procedure
文章信息
稿件历史记录
提交日期: Aug 19, 2024
接收日期: Oct 25, 2024
在线发布日期: Nov 14, 2024
出版日期: Jan 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Hartveit, E. and Veruki, M. L. (2025). Capacitance Measurements of Exocytosis From AII Amacrine Cells in Retinal Slices. Bio-protocol 15(1): e5147. DOI: 10.21769/BioProtoc.5147.
Hartveit, E., Veruki, M. L. and Zandt, B. J. (2022). Dendritic Morphology of an Inhibitory Retinal Interneuron Enables Simultaneous Local and Global Synaptic Integration.J Neurosci. 42(9): 1630–1647.
分类
神经科学 > 细胞机理 > 突触生理学
生物物理学 > 电生理 > 膜片钳技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link