- EN - English
- CN - 中文
A Novel and Robust Method for Investigating Fungal Biofilm
真菌生物膜研究的新型稳健方法
发布: 2025年01月05日第15卷第1期 DOI: 10.21769/BioProtoc.5146 浏览次数: 1766
评审: Lucy XieSascha BrunkeSimab KanwalShailesh Kumar
Abstract
Candida auris, labeled an urgent threat by the CDC, shows significant resilience to treatments and disinfectants via biofilm formation, complicating treatment/disease management. The inconsistencies in biofilm architecture observed across studies hinder the understanding of its role in pathogenesis. Our novel in vitro technique cultivates C. auris biofilms on gelatin-coated coverslips, reliably producing multilayer biofilms with extracellular polymeric substances (EPS). This method, applicable to other Candida species like C. glabrata and C. albicans, is cost-effective and mimics the niche of biofilm formation. It is suitable for high-throughput drug screening and repurposing efforts, aiding in the development of new therapeutics. Our technique represents a significant advancement in Candida biofilm research, addressing the need for consistent, reproducible biofilm models. We detail a step-by-step procedure for creating a substratum for biofilm growth and measuring biofilm thickness using confocal laser scanning microscopy (CLSM) and ultrastructure by scanning electron microscopy (SEM). This method provides consistent outcomes across various Candida species.
Key features
• The biofilm formed on gelatin surfaces mimics host conditions, replicating the multilayered structure and EPS, offering a more accurate model for studying C. auris biofilms.
• This method is highly reproducible and suitable for drug screening and biofilm analysis through three-dimensional (3D) reconstruction.
• This in vitro technique aids in studying biofilm formation, related virulence properties, and drug tolerance of C. auris and other Candida species.
• The simple, cost-effective technique is ideal for screening novel inhibitors and repurposed drug libraries, facilitating the design/identification of new therapeutics against Candida species.
Keywords: Candida albicans (白假丝酵母)Graphical overview
Background
Candidiasis is a serious threat, especially to individuals with compromised immune systems and comorbidities. Hospitalized patients, particularly those who have undergone organ or bone marrow transplants or are receiving immune-suppressive therapies, are highly susceptible to infections [1]. Prolonged hospital stays further increase the risk of contracting hospital-acquired infections [2]. A critical concern is the emergence of Candida auris, a fungal pathogen first isolated in 2009 [3]. Since then, C. auris has become a global health concern, with reports of drug resistance emerging from as many as 32 countries, particularly during the COVID-19 pandemic among hospitalized patients [4]. C. auris as a drug-resistant nosocomial pathogen has a reported mortality rate of 30%–50% in infected persons [5]. This pathogen can cause various infections, including ear, systemic (fungemia), wound, lung, urinary tract, and gut infections [5–7]. Due to its high resistance to various antifungal treatments and its potential for pan-drug resistance, the World Health Organization (WHO) has listed C. auris in the critical priority group of fungal pathogen priority list (FPPL) released in October 2022 [8].
Unlike other Candida species, C. auris seldom colonizes the gut but can persist on the skin by forming resilient biofilms, which causes rapid spread of this fungus in hospitals [9]. C. auris demonstrates remarkable resilience, especially in its biofilm form, which shows substantial resistance [10]. According to the Centers for Disease Control and Prevention (CDC), C. auris biofilms are resistant to common disinfectants, including ammonium salt–based solutions, and can endure harsh conditions [11]. C. auris can persist in the cutaneous layer by dividing and forming biofilms, which can cause bloodstream infection when they reach the blood vessels [12]. This underscores the necessity of studying biofilm formation in infection scenarios to develop effective treatments. Given the widespread drug resistance, there is an urgent need for robust screening platforms to identify novel inhibitors. Drug repurposing must be validated through rigorous in vitro and in vivo screening methods. However, a major challenge is establishing a reliable method to form biofilm models that accurately replicate in vivo conditions. The phenotypes of C. auris biofilms can vary significantly based on culture conditions, complicating research outcomes. Current methods, such as those using Thermanox (polystyrene) coverslips or porcine skin surfaces [13,14], either fail to produce typical biofilm architecture or lack cost-effectiveness for large-scale screenings. A standard surface for biofilm proliferation is crucial for reproducibility, as effective drug screening requires the typical multilayered architecture of biofilms.
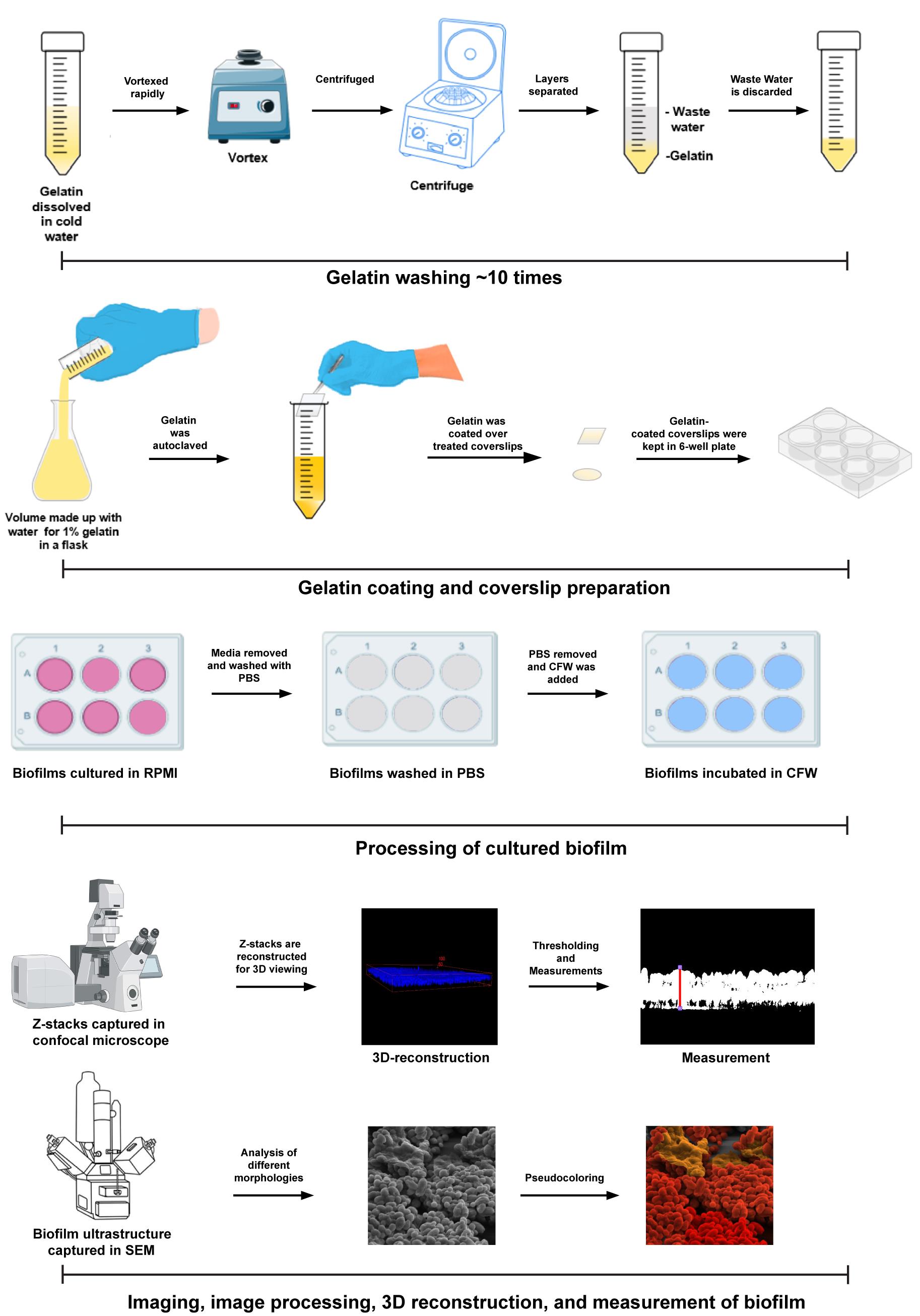
To address these issues, we proposed a novel in vitro method for growing C. auris biofilms on gelatin-coated coverslips derived from bovine skin. Gelatin, a derivative of collagen, mimics the matrix of mammalian cells, thereby more closely replicating natural conditions. This method successfully forms heterogeneous, multilayered biofilms of C. auris. The architecture of these biofilms has been analyzed using scanning electron microscopy (SEM) and compared with previous studies [14–16]. Furthermore, the versatility and accuracy of this method have been demonstrated using confocal scanning laser microscopy (CSLM) to measure biofilm thickness. The application of this innovative in vitro technique is not only limited to C. auris biofilms but can also be used to study biofilm formation in other Candida species, such as Candida glabrata and Candida albicans. This method is a potent, cost-effective solution for large-scale drug screenings.
Here, we describe a stepwise procedure for creating a substratum for biofilm growth and measuring biofilm thickness with confocal laser scanning microscopy and ultrastructures by scanning electron microscopy. As mentioned above, it results in reliable outcomes and is suitable for various Candida species with different media. This method has been successfully used in previously published studies, demonstrating its effectiveness and reliability [15,16].
Materials and reagents
Biological materials
1. Candida auris strain of clade 2, CBS10913T
Reagents
1. Gelatin derived from bovine skin and bones (Sigma-Aldrich, catalog number: G9391-100G)
2. Double-distilled water
3. BD Difco YPD broth (Fisher Scientific, catalog number: DF0428-17-5)
4. 0.3 N hydrochloric acid (HCl) (Fisher Scientific, catalog number: 7647-01-0)
5. 100% ethanol (G-Bioscience, catalog number: RC1533)
6. 3-aminopropyl triethoxysilane (Himedia, catalog number: RM6592-100G)
7. Acetone HPLC grade (MERCK, catalog number: 60002010001730)
8. Glutaraldehyde 25%, w/w (Himedia, catalog number: MB222-100ML)
9. RPMI-1640 w/ or w/o MOPS and with L-glutamate added (Himedia, catalog number: AL028G-500ML)
10. Calcofluor white (CFW) (Sigma-Aldrich, catalog number: 18909-100ML-F)
11. Paraformaldehyde (Sigma-Aldrich, catalog number: F8775-500ML)
12. Alcian blue (Himedia, catalog number: RM471-25G)
13. Sodium cacodylate trihydrate (Himedia, catalog number: RM3732-25G)
14. Sodium chloride (NaCl) (Himedia, catalog number: MB023-1KG)
15. Potassium chloride (KCl) (G Biosciences, catalog number: RC1167)
16. Sodium phosphate dibasic anhydrous (Na2HPO4) (SRL, catalog number: 53046)
17. Potassium phosphate monobasic (KH2PO4) (G Biosciences, catalog number: RC1173)
18. Transparent nail paint
Solutions
1. YPD broth (see Recipes)
2. 1× PBS (see Recipes)
3. Scanning electron microscopy (SEM) buffer (see Recipes)
4. Other solutions (see Recipes)
Recipes
1. YPD broth (1 L)
Add 50 g of Difco YPD broth to 1 L of distilled water and sterilize by autoclaving at 121 °C for 20 min before use.
2. 1× PBS (1 L)
To prepare 1× PBS, weigh the components mentioned in the table below and dissolve in 500 mL of water. Once all the components are dissolved, make up the volume to 1 L and autoclave.
| Reagent | Amount |
|---|---|
| NaCl | 8 g |
| KCl | 0.2 g |
| Na2HPO4 | 1.44 g |
| KH2PO4 | 0.24 g |
| Double-distilled water (ddH2O) | to 1 L |
3. Scanning electron microscopy (SEM) buffer (50 mL)
To prepare the SEM buffer, add the following chemicals and make up the volume to 50 mL. Filter with pore size 0.22 μm, sterilize, and store at -20 °C.
| Reagent | Stock concentration | Amount | Final concentration |
|---|---|---|---|
| Alcian blue | n/a | 75 mg | 0.15% |
| Sodium cacodylate | n/a | 1.2 g | 0.15 M |
| Glutaraldehyde | 25% | 4 mL | 2% |
| Paraformaldehyde | 37% | 2.7 mL | 2% |
| Double distilled water (ddH2O) | n/a | To 50 mL | n/a |
| Total | 50 mL |
4. Other solutions
| Reagents | Stock concentration | Amount | Final concentration |
|---|---|---|---|
| CFW | 1 mg/mL | 30 μL | 30 μg/mL in 1 mL |
| HCl | 12 N | 2.5 mL | 0.3 N in 100 mL |
| 3-aminopropyl triethoxysilane | n/a | 1 mL | 2% in 50 mL |
| Gelatin | n/a | 1g | 1% in 100 mL |
| Ethanol | 100% | 2.5 mL (10 mL) | 25% |
| 100% | 5 mL (10 mL) | 50% | |
| 100% | 7 mL (10 mL) | 70% | |
| 100% | 8 mL (10 mL) | 80% | |
| 100% | 9.5 mL (10 mL) | 95% |
Laboratory supplies
1. 1.5 mL microcentrifuge tubes (Tarsons, catalog number: 500010)
2. 15 mL conical bottom tubes (Tarsons, catalog number: 430766)
3. 50 mL conical bottom tubes (Tarsons, catalog number: 430829)
4. 1,000 μL tips (Tarsons, catalog number: 521020B)
5. 200 μL tips (Tarsons, catalog number: 521010Y)
6. Conical flasks (Borosil catalog number: 4980021)
7. 90 mm Petri dishes (Tarsons, catalog number: 460095)
8. 24-well polystyrene plates (Thermo Scientific, catalog number: 142475)
9. 6-well polystyrene plates (Thermo Scientific, catalog number: 140675)
10. Kimwipes (Fisher Scientific, catalog number: 34120)
11. Glass slides (any high-quality glass slides)
12. Glass coverslips (any high-quality glass coverslips)
13. Carbon tape (TED PELLA, Inc., product no.: 16084-1)
Equipment
1. Eppendorf New Brunswick InnovaTM 42/42 R, stackable incubator shaker (Fisher Scientific, catalog number: 05-400-162)
2. Heat block (DTH-100 Dry bath incubator)
3. Vortex (BR Biochem, catalog number: BI-VM-2500)
4. Refrigerated benchtop centrifuge 5910 R (Eppendorf, catalog number: 5942000130)
5. Centrifuge 5427 R (Eppendorf, catalog number: 5429000133)
6. Light microscope (Leica, model: Sp5)
7. Scanning electron microscope (Apreo VolumeScope FEI)
8. Airstream® class II type A2 biological safety cabinet
9. Spectrophotometer Gene Quant 1300 (VWR, catalog number: SCLI80-2120-02)
10. Vacuum desiccator (Sigma-Aldrich, catalog number: Z119024)
11. Quorum SC7620 sputter coater
12. Hot-air oven (Scientific Systems)
13. Digital orbital shaker (Heathrow Scientific, catalog number: 120460)
14. Forceps
15. Autoclave
16. Water bath (Grant instruments, model: SBB Aqua 5 plus)
Software and datasets
1. LAS-AF
2. FIJI 1.54
3. GraphPad Prism 9
4. Adobe Photoshop CC 2019
Procedure
文章信息
稿件历史记录
提交日期: Jul 5, 2024
接收日期: Oct 23, 2024
在线发布日期: Nov 20, 2024
出版日期: Jan 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Biswas, B., Asif, S., Puria, R. and Thakur, A. (2025). A Novel and Robust Method for Investigating Fungal Biofilm. Bio-protocol 15(1): e5146. DOI: 10.21769/BioProtoc.5146.
分类
微生物学 > 微生物生物膜 > 生物膜培养
生物科学 > 微生物学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link