- EN - English
- CN - 中文
Cryopreservation Method for Preventing Freeze-Fracture of Small Muscle Samples
防止小肌肉样本冻裂的冷冻保存方法
发布: 2025年01月05日第15卷第1期 DOI: 10.21769/BioProtoc.5145 浏览次数: 1945
评审: Vivien J. Coulson-ThomasThirupugal GovindarajanAnonymous reviewer(s)
Abstract
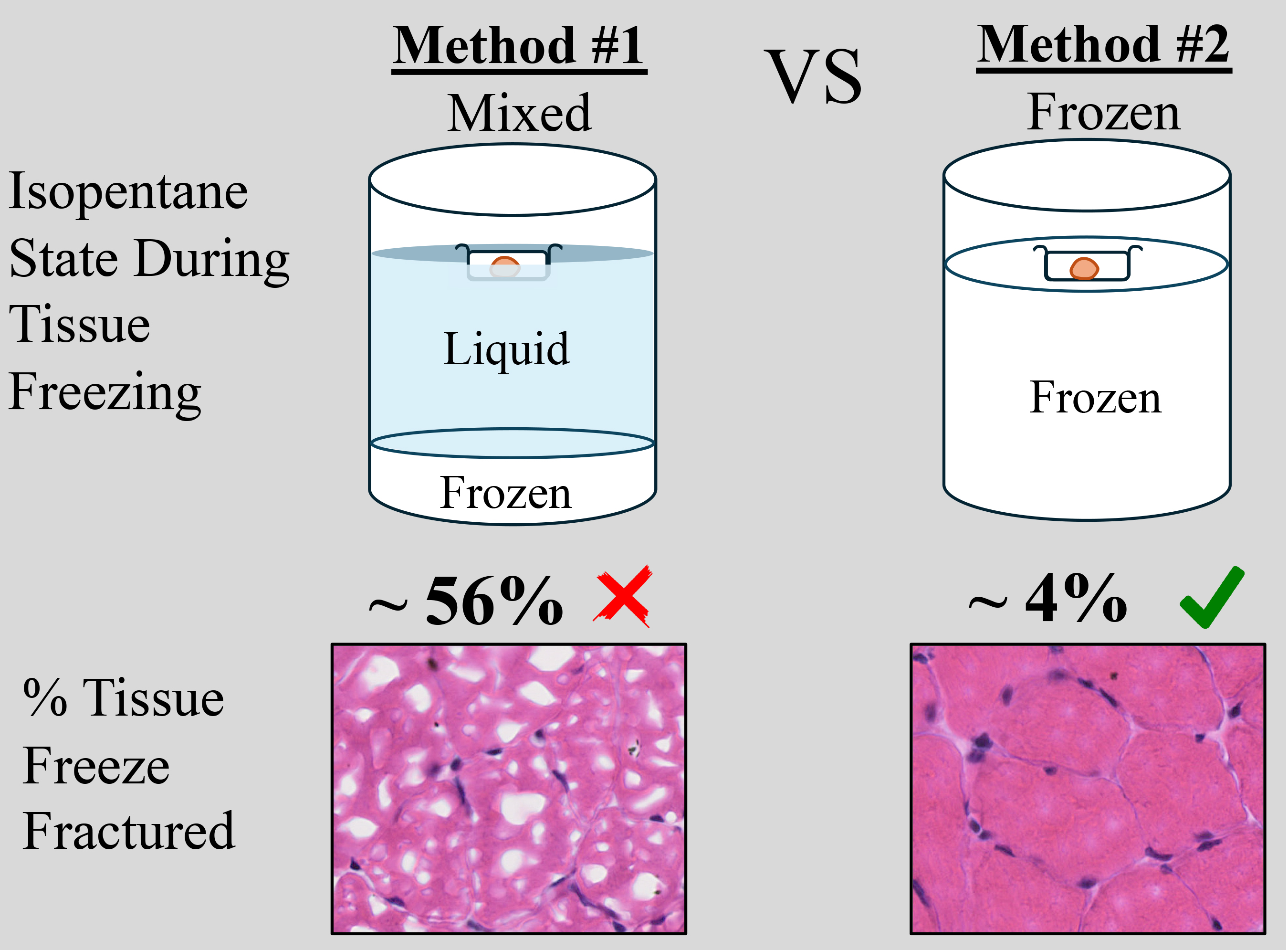
Histological techniques to study muscle are crucial for assessing skeletal muscle health. To preserve tissue morphology, samples are usually fixed in formaldehyde or cryopreserved immediately after excision from the body. Freezing samples in liquid nitrogen, using isopentane as a mediator for efficient cooling, preserves the tissue in its natural state. However, this method is highly susceptible to freeze-fracture artifacts, which alter or destroy tissue architecture. Isopentane is most commonly used in a semi-frozen/liquid state that is visually assessed by the experimenter, which can pose a challenge when freezing multiple tissues at a time or maintaining a consistent temperature. Furthermore, tissue size is also a confounding factor; depending on the size, freezing times can vary. In this study, we compare two different options for using isopentane while cryopreserving tissue. We also present an easy and reproducible method of freezing the soleus tissue of mice using frozen isopentane. This method decreased the occurrence of freeze-fractures by an order of magnitude, to ~4%, whereas the traditional method of cryopreservation resulted in ~56% freeze-fracturing.
Key features
• A uniform and highly reproducible protocol for freezing any tissue that is prone to freeze-fracture.
• Removes the need to maintain a mixed state of isopentane.
• Optimized cryopreservation method for the soleus muscle of mice.
• Allows for prevention of peripheral freeze-fracture in tissue, which is the most susceptible region to freeze-fracture damage.
Keywords: Freeze-fracture (冻裂)Graphical overview
Background
Preserving tissue integrity is paramount for comprehensive morphological studies. Previous methods employed for tissue preservation, including formaldehyde fixation and cryopreservation, have demonstrated both advantages and challenges [1]. Formaldehyde fixation, while stabilizing tissues through protein crosslinking, may alter the natural conformation of cellular structures and impede antigen accessibility during immunohistochemical analysis. Cryopreservation, involving rapid freezing in liquid nitrogen without the need for fixation, is a widely used method for long-term tissue storage. However, the sudden temperature drop associated with liquid nitrogen often distorts the morphology of skeletal muscles. The liquid nitrogen evaporates immediately as it comes into contact with the warm tissue. This vapor becomes trapped between the mold surface and the liquid nitrogen, essentially creating an insulated barrier preventing freezing [2]. This process creates artifacts where intracellular water crystallizes immediately, resulting in the formation of huge crystals that rupture the muscle fibers. To address this, isopentane is utilized as a medium to facilitate controlled freezing [3].
A beaker filled with isopentane is placed in liquid nitrogen such that one-third of the isopentane is submerged. The isopentane is considered good for cryopreservation when a frozen layer forms at the bottom. At this stage, the bottom of the tissue mold is submerged in the chilled liquid. The duration of tissue submersion varies depending on the size of the tissue. Larger tissue requires longer freezing times than smaller tissues. The freezing times for small tissues like the diaphragm or extensor digitorum longus (EDL) is 6–12 s [3]. However, mice have even smaller tissues (e.g., soleus) that would require a freezing time of less than 6 s. Hence, small skeletal muscle tissues remain susceptible to freeze-fractures due to their higher surface-to-volume ratio. This method of freezing is highly inconsistent due to the many variables, i.e., liquid-to-frozen phase change, temperature fluctuations, visual assessment of phase state, tissue size, submerged depth of the mold, and batch-to-batch variability. In this study, rather than visual assessment, we monitor the temperature of isopentane when used for cryopreservation and present a reliable and reproducible method of preserving mouse skeletal muscles for both large and small tissues.
Materials and reagents
Biological materials
1. Tibialis anterior and soleus from 6-week-old C57BL/6 males (Jackson Laboratory, strain #000664)
Reagents
1. Bendable thermocouple probes for liquids and gases (McMaster Carr, catalog number: 39095K96)
2. 2-methylbutane/isopentane (Sigma-Aldrich, catalog number: 277258)
3. Dry ice
4. Liquid nitrogen
5. OCT (Optimal Cutting Temperature) (Sakura, catalog number: 4583)
6. CitriSolv (Fisher Scientific, catalog number: 04-355-121)
7. Bluing reagent (Fisher Scientific, catalog number: 22-050-114, Supplier No.: 7301)
8. Formalin (Fisher Scientific, catalog number: SF100-20)
9. Ethanol (Decon Laboratories, Inc, catalog number: 2701)
10. Hydrochloric acid (HCl) (Fisher Scientific, catalog number: A144-500)
11. Hematoxylin 560-Surgipath (Leica Biosystems, catalog number: 3801570)
12. Alcoholic eosin Y515-Suripath (Leica Biosystems, catalog number: 3801615)
Laboratory supplies
1. Modified big aluminum can (23Fl Oz), see section B: Cryopreservation method #1
2. Modified small aluminum can (11.5Fl Oz), see section C: Cryopreservation method #2
3. Handheld thermometer (McMaster Carr, catalog number: 9281T52)
4. Styrofoam ice box
5. Tissue molds (VWR, catalog number: 25608-916, Supplier No.: 4557)
6. Needles 23G (Exel International, catalog number: 26407)
7. Microscope slides (Epic Scientific, catalog number: 187200810RE)
8. Superfrost plus slides (Fisher Scientific, catalog number: 1255015)
Equipment
1. Blunt and sharp forceps (Fisher Scientific, catalog numbers: 12740916 and 11340705)
2. Scissors (Fisher Scientific, catalog number: 08-940)
3. Locking forceps (Fisher Scientific, catalog number: 13-812-16)
4. Cryostat blade (C.L. Sturkey, Inc. catalog number: 22-210-045)
5. Nanozoomer (Hamamatsu, model: C9600-12, catalog number: 000382)
Software and datasets
1. NDPI viewer software (Hamamatsu version 2.9.29)
Procedure
文章信息
稿件历史记录
提交日期: Aug 1, 2024
接收日期: Oct 18, 2024
在线发布日期: Nov 12, 2024
出版日期: Jan 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Ghag, N., Tam, J., Anderson, R. R. and Cheema, N. (2025). Cryopreservation Method for Preventing Freeze-Fracture of Small Muscle Samples. Bio-protocol 15(1): e5145. DOI: 10.21769/BioProtoc.5145.
分类
细胞生物学 > 细胞分离和培养 > 低温贮存
细胞生物学 > 组织分析 > 组织形态学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












