- EN - English
- CN - 中文
Temporally and Spatially Controlled Age-Related Prostate Cancer Model in Mice
小鼠时间与空间特异性控制的前列腺癌模型
(*contributed equally to this work) 发布: 2025年01月05日第15卷第1期 DOI: 10.21769/BioProtoc.5144 浏览次数: 2685
评审: Anonymous reviewer(s)
Abstract
The initiation and progression of prostate cancer (PCa) are associated with aging. In the history of age-related PCa research, mice have become a more popular animal model option than any other species due to their short lifespan and rapid reproduction. However, PCa in mice is usually induced at a relatively young age, while it spontaneously develops in humans at an older age. Thus, it is essential to develop a method by which the PCa initiation and progression timeline can be strictly controlled to mimic human physiological conditions. One milestone in this field was the identification of the prostate-specific transcription factor, Probasin (Pb), which allowed for the prostate-specific expression of genes knocked into the mice's genome. Another milestone is the establishment of the preclinical mouse model with Pten conditionally knocked out in the prostate tissue, which closely mimics the formation and growth of human PCa. Hereby, we present the prostate-specific temporally and spatially controlled Pten knockout PCa mouse model that can be induced using an adenovirus-based Cre-LoxP system. The Cre recombinase (Cre) is inserted into an adenovirus vector. Unlike Pb-Cre knock-in models (which are spatially but not temporally controlled), the expression of Cre is activated to knock out Pten from the mice's prostate epithelial cells once injected. The viral delivery procedures strictly control the location and time of Pten knockout. This novel approach provides a powerful age-related murine model for PCa, emphasizing the effect of aging on prostate carcinogenesis.
Key features
• In vivo delivery of Cre recombinase adenovirus (Ad-Cre-Luc) in Pten LoxP/LoxP (L/L) mice.
• Generation of Cre-expressing Ad-Cre-Luc-mediated ablation of Pten in anterior prostate epithelial cells of adult Pten L/L mice at different ages.
• The Ad-Cre-Luc-mediated ablation of Pten leads to hyperplasia that progresses through prostatic intraepithelial neoplasia (PIN) to adenocarcinoma.
• PIN refers to the non-cancerous growth of epithelial cells in the prostate tissue—not cancer but a precursor of prostate cancer [1].
Keywords: In vivo viral delivery (体内病毒递送)Graphical overview
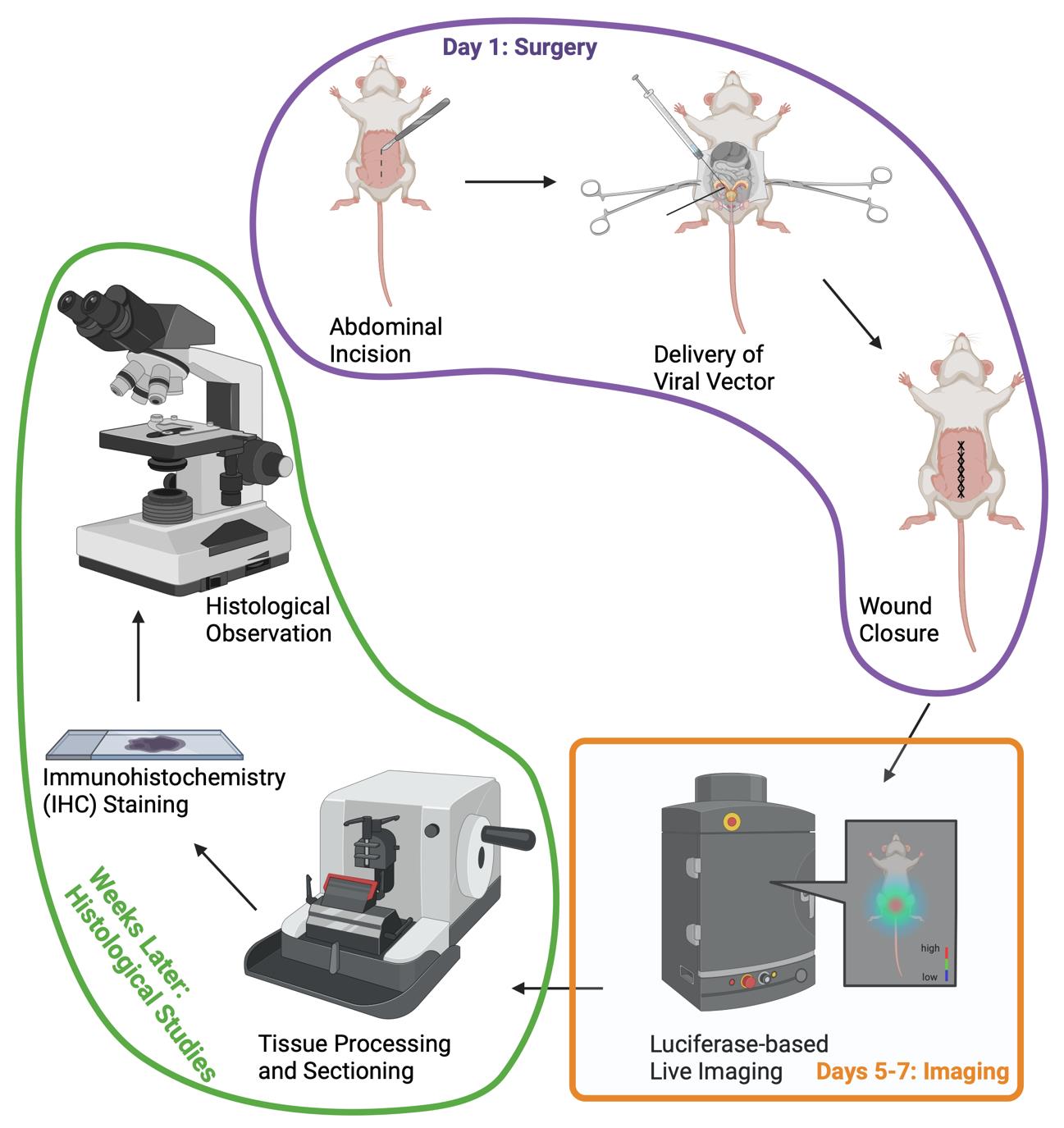
Surgery, luciferase-based bioluminescent imaging, and histological observation
Background
Among all risk factors, age is the most important one associated with prostate cancer (PCa) [2]. Canine models are considered valuable in preclinical studies of PCa because they are similar to humans in terms of size, anatomy, histology, and spontaneity of PCa [3]. However, the relatively long lifespan and low incidence rate may limit the use of these models, especially in age-related studies [3]. In contrast, mice's rapid reproduction and short lifespan make them ideal models in which the role of aging in PCa can be studied relatively quickly. However, PCa in mice models must be induced, which is less likely to resemble human conditions, especially the age of PCa initiation. Hence, there is an urgent need to develop a murine model where the timeline of PCa initiation and progression can be strictly controlled.
A milestone in this field is the creation of the prostate-specific Pten conditional knockout (cKO) mouse model, which closely mimics the formation and growth of human PCa and has become an established PCa preclinical model [4]. However, as Pten deletion in this model is an automatic process triggered in very young prostate tissue (less than eight weeks old), it is challenging to mimic the physiology and the microenvironment (old prostate tissue) in which human PCa develops. We created a novel mouse model where Pten KO can be spatially and temporally controlled (Pten adcre+) using a virus-assisted in vivo cKO approach. The prostate-specific Cre-LoxP gene switching was generated via intraductal delivery of adenovirus to the anterior prostate lobes [5]. Adenovirus is a DNA virus that does not integrate into the host genome. It infects dividing and nondividing cells, leading to a transient high-level protein expression [6]. Using the intraductal delivery method, we obtained epithelial-specific prostate infection with Cre-expressing adenoviruses, leading to the deletion of the floxed Pten gene in the prostate epithelium.
Materials and reagents
Biological materials
1. Ptenflox mice (The Jackson Laboratory, strain number: 006440)
2. Cre recombinase adenovirus (Ad-Cre-Luc) (VECTOR BIOLABS, catalog number: 1705); co-expression of Cre recombinase and luciferase (the reporter gene) is regulated by the cytomegalovirus (CMV) promoter
3. Luciferase adenovirus (Ad-CMV-Luc) (VECTOR BIOLABS, catalog number: 1000); this adenovirus vector delivers luciferase. Expression is also regulated by the CMV promoter. It is used as a Cre-negative control of the Ad-Cre-Luc viral vector
Reagents
1. Lidocaine 2% (VETONE, catalog number: V1 510212)
2. OstiLOX (VETONE, catalog number: V1 501080)
3. Sodium chloride (NaCl) (Fisher Chemical, catalog number: S271-10)
4. 10% povidone iodine (McKesson, catalog number: MFR#036)
5. Paraformaldehyde (Sigma-Aldrich, catalog number: P6148-1KG)
6. 100% ethanol, anhydrous (Fisher Chemical, catalog number: A405P-4)
7. Xylene (Thermo Scientific, catalog number: 9990501)
8. CAT hematoxylin (BIOCARE MEDICAL, catalog number: CATHE-GL)
9. Edgar degas eosin (BIOCARE MEDICAL, catalog number: THE-GL)
10. Tacha's bluing solution 10× (BIOCARE MEDICAL, catalog number: HTBLU-MX)
11. Saturated HCl (Fisher Scientific, catalog number: 60047420)
12. Soothe eye ointment (Bausch & Lomb, Preservative Free, 1.8 Oz)
13. Blocking serum from VECTASTAIN Elite ABC Kit (VECTOR LABORATORIES INC, catalog number: PK-6101); see Recipes
14. Biotinylated secondary antibody from VECTASTAIN Elite ABC Kit (VECTOR LABORATORIES INC, catalog number: PK-6101); see Recipes
15. 3,3'-diaminobenzidine (DAB) solution (VECTOR LABORATORIES INC, catalog number: sk-4100); see Recipes for final concentration
16. Phosphate-buffered saline (PBS) (GENESEESCI, catalog number: 25-508)
17. 30% hydrogen peroxide (H2O2) (Fisher Scientific, catalog number: H254-500)
18. D-luciferin (Cayman Chemical Company, catalog number: 14682)
Solutions
1. OstiLox for injection (see Recipes)
2. Lidocaine for injection (see Recipes)
3. 10% paraformaldehyde (see Recipes)
4. 15% ethanol (see Recipes)
5. 70% ethanol (see Recipes)
6. 80% ethanol (see Recipes)
7. 95% ethanol (see Recipes)
8. Bluing solution (see Recipes)
9. 0.5% HCl-ethanol solution (see Recipes)
10. Blocking serum from VECTASTAIN Elite ABC Kit (see Recipes)
11. Biotinylated secondary antibody from VECTASTAIN Elite ABC Kit (see Recipes)
12. Avidin-Biotin-peroxidase Complex (ABC) solution from VECTASTAIN Elite ABC Kit (see Recipes)
13. DAB solution (see Recipes)
Recipes
1. OstiLox for injection
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| OstiLox | 1 mg/mL | 200 μL |
| NaCl | 9 mg/mL | 800 μL |
| Total | n/a | 1 mL |
2. Lidocaine for injection
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Lidocaine 2% | 4 mg/mL | 200 μL |
| NaCl | 9 mg/mL | 800 μL |
| Total | n/a | 1 mL |
3. 10% paraformaldehyde
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Paraformaldehyde | 100 g/L | 100 g |
| PBS | 1× | 1 L |
| Total | n/a | 1 L |
4. 15% ethanol
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ethanol anhydrous | 15% (v/v) | 150 mL |
| Deionized H2O | n/a | Adjust the total volume to 1 L |
| Total | n/a | 1 L |
5. 70% ethanol
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ethanol anhydrous | 70% (v/v) | 700 mL |
| Deionized H2O | n/a | Adjust the total volume to 1 L |
| Total | n/a | 1 L |
6. 80% ethanol
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ethanol anhydrous | 80% (v/v) | 800 mL |
| Deionized H2O | n/a | Adjust the total volume to 1 L |
| Total | n/a | 1 L |
7. 95% ethanol
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ethanol anhydrous | 95% (v/v) | 950 mL |
| Deionized H2O | n/a | Adjust the total volume to 1 L |
| Total | n/a | 1 L |
8. Bluing solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tacha's bluing solution 10× | 1× | 100 mL |
| Deionized H2O | n/a | Adjust the total volume to 1 L |
| Total | n/a | 1 L |
9. 0.5% HCl-ethanol solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Saturated HCl | 0.5% (v/v) | 1 mL |
| 70% ethanol | 1× | 199 mL |
| Total | n/a | 200 mL |
10. Blocking serum from VECTASTAIN Elite ABC Kit
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Blocking serum | 1.5% (v/v) | 150 μL |
| PBS | 1× | 10 mL |
| Total | n/a | 10.15 mL |
11. Biotinylated secondary antibody from VECTASTAIN Elite ABC Kit
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Biotinylated antibody | 1:200 diluted | 50 μL |
| Blocking serum | 1.5% (v/v) | 150 μL |
| PBS | 1× | 10 mL |
| Total | n/a | 10.2 mL |
12. ABC solution from VECTASTAIN Elite ABC Kit
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Avidin DH | 1:50 diluted | 100 μL |
| Biotinylated horseradish peroxidase H | 1:50 diluted | 100 μL |
| PBS | 1× | 5 mL |
| Total | n/a | 5.2 mL |
13. DAB solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PBS | 0.0152× | 15.2 μL |
| DAB | 0.5 g/L | 17.6 μL |
| H2O2 | 0.15 g/L | 16 μL |
| Deionized H2O | n/a | Adjust the total volume to 1 mL |
| Total (optional) | n/a | 1 mL |
Laboratory supplies
1. Microscope slides (Fisher Scientific, catalog number: 12-550-15)
2. Cover glass (Brain Research Laboratories, catalog number: 2222-1.5D)
3. Tissue cassettes with recessed cover (Simport Scientific, catalog number: M509-2)
4. 4-Ply non-woven, non-sterile dental gauze sponges (PlastCare USA, catalog number: PG‐3304‐3)
5. GLAD PRESS'N SEAL food wrap (Phoenix Research Industries INC, catalog number: CLO70441)
6. 5-0 nylon sutures (Securos Surgical, catalog number: CG634)
7. SimPort tissue cassette (SimPort Scientific INC, catalog number: M-509-2)
8. ImmEdge pen (VECTOR LABORATORIES INC, catalog number: H-4000)
9. Coverslip 22 × 22 (Fisher Scientific, catalog number: C10228)
Equipment
1. Bead sterilizer: The germ terminator (CellPoint scientific, model: GERMINATORTM 500)
2. Biological safety cabinet (The Bader Company, catalog number: SG404)
3. Surgipath Paraplast surgical microscope (Leica, catalog number: 39601006)
4. Gas anesthesia system (Caliper LifeScience, model: XGI-8)
5. IVIS-Lumina (Caliper LifeScience, model: XRMS)
6. Microtome (New Life Scientific INC, model: HM355S)
7. Isotemp incubator (Fisher Scientific, model: 637D)
8. Nikon microscope (PRECISION INSTRUMENTS, LLC, model: H550S)
9. 50 μL, model 705 RN syringe (Hamilton, catalog number: CAL7637-01)
10. 33 gauge, small hub RN needle, custom length (0.5 to 12 in.), point style 2, 3 or 4, 6/PK (Hamilton, catalog number: 7803-05)
11. Decloaking ChamberTM NxGen (BIOCARE MEDICAL, model: DC2012)
12. Sterile surgical tools (forceps, scalpel, small scissors, and hemostats)
13. Micropipette (Hamilton, catalog number: 7637-01)
Software and datasets
1. PureCLIP (Sabrina Krakau et al., https://github.com/skrakau/PureCLIP) [7]
2. NIS-Elements BR Analysis (Nikon, 4.60.00, https://www.microscope.healthcare.nikon.com/products/software/nis-elements/nis-elements-basic-research)
Procedure
文章信息
稿件历史记录
提交日期: Jul 19, 2024
接收日期: Oct 23, 2024
在线发布日期: Nov 11, 2024
出版日期: Jan 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Liu, S., Shen, K., Li, Z., Rivero, S. and Zhang, Q. (2025). Temporally and Spatially Controlled Age-Related Prostate Cancer Model in Mice. Bio-protocol 15(1): e5144. DOI: 10.21769/BioProtoc.5144.
分类
癌症生物学 > 通用技术 > 动物模型
分子生物学 > DNA > 基因表达
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link