- EN - English
- CN - 中文
Muscle Biopsy Sample Preparation and Proteomics Analysis Based on UHPLC-MS/MS
肌肉活检样本制备及基于UHPLC-MS/MS的蛋白质组学分析
(*contributed equally to this work) 发布: 2024年12月20日第14卷第24期 DOI: 10.21769/BioProtoc.5137 浏览次数: 1912
评审: Amit Kumar DeyAnonymous reviewer(s)

相关实验方案

适用于 LC–MS/MS 蛋白质组学分析的大体积细胞培养上清分泌组样品制备方法优化
Basil Baby Mattamana [...] Peter Allen Faull
2025年12月20日 1213 阅读
Abstract
Proteomics analysis is crucial for understanding the molecular mechanisms underlying muscle adaptations to different types of exercise, such as concentric and eccentric training. Traditional methods like two-dimensional gel electrophoresis and standard mass spectrometry have been used to analyze muscle protein content and modifications. This protocol details the preparation of muscle samples for proteomics analysis using ultra-high-performance liquid chromatography (UHPLC). It includes steps for muscle biopsy collection, protein extraction, digestion, and UHPLC-based analysis. The UHPLC method offers high-resolution separation of complex protein mixtures, providing more detailed and accurate proteomic profiles compared to conventional techniques. This protocol significantly enhances sensitivity, reproducibility, and efficiency, making it ideal for comprehensive muscle proteomics studies.
Key features
• Developed for analyzing muscle adaptations in response to concentric and eccentric training, applicable to various physiology exercise studies.
• Utilizes UHPLC-MS/MS for high-resolution separation and detailed proteomic profiling.
• Requires access to advanced UHPLC-MS/MS equipment and muscle biopsy collection tools.
• The protocol can be completed within one week, including sample preparation and analysis.
Keywords: Muscle proteomics (肌肉蛋白质组学)Graphical overview
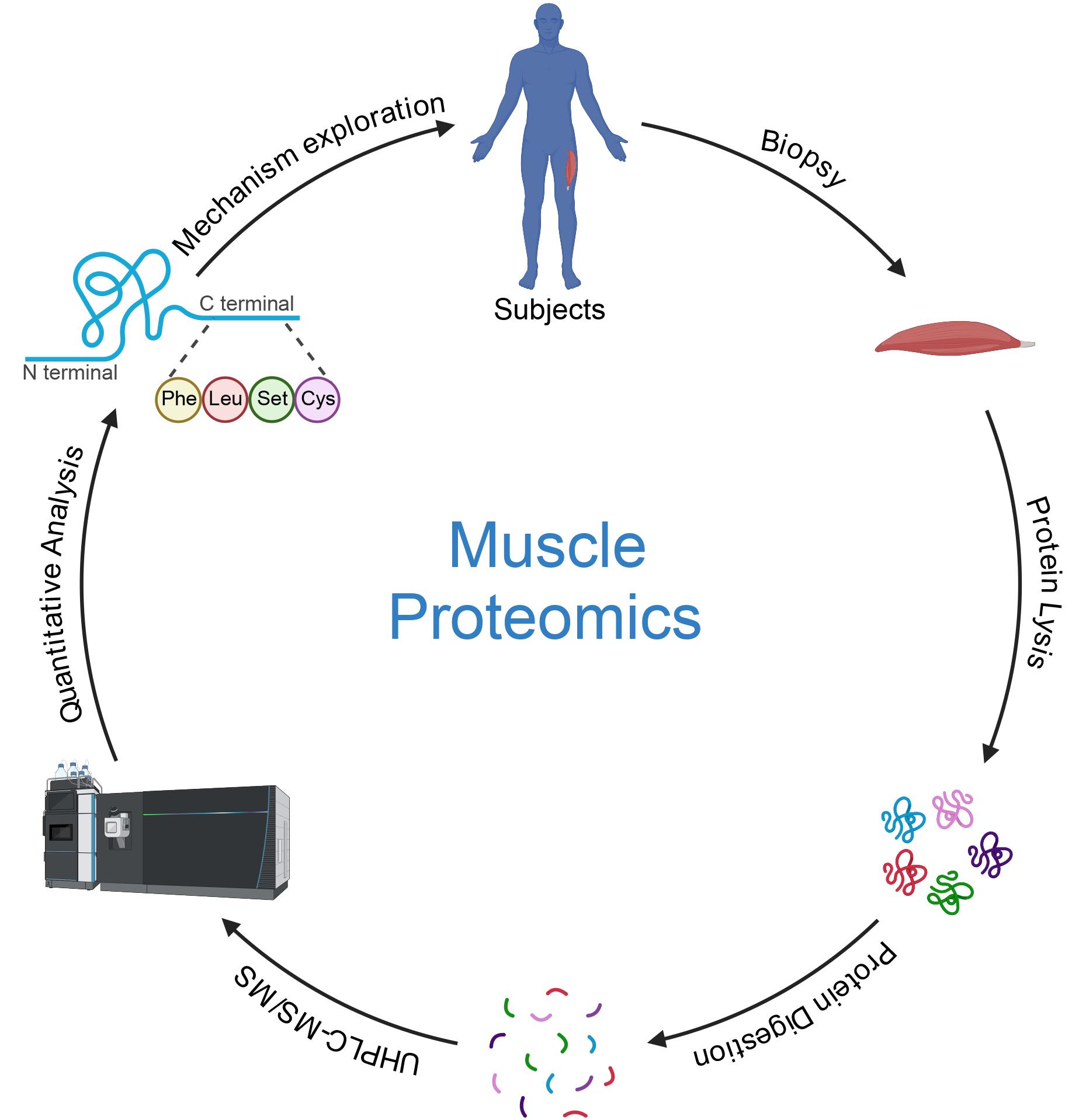
Background
Proteomics is an essential field in biomedical research, offering deep insights into the molecular mechanisms underlying various physiological and pathological processes [1]. This protocol focuses on muscle proteomics, specifically analyzing muscle adaptations to different types of exercise, such as concentric (CON) and eccentric (ECC) training. Muscle proteomics can elucidate the intricate molecular changes that occur in response to physical activity, thereby advancing our understanding of exercise physiology, muscle hypertrophy, and recovery [2]. Traditional liquid chromatography–mass spectrometry (LC–MS) techniques have long been employed for proteomic analysis, offering significant advantages over two-dimensional gel electrophoresis in terms of sensitivity and reproducibility [3]. However, the separation power of conventional LC systems often falls short when dealing with complex mixtures like muscle tissue proteomes, where resolving peptides with close retention times and molecular weights is crucial [4].
The protocol described here utilizes ultra-high-performance liquid chromatography (UHPLC) for the preparation and analysis of muscle samples. This method offers several significant advantages over traditional approaches. UHPLC provides superior resolution and separation of complex protein mixtures compared to conventional liquid chromatography, allowing for more detailed and accurate proteomic profiling [5]. The protocol significantly increases the sensitivity of protein detection, enabling the identification of low-abundance proteins that might be missed using standard methodologies [6]. Additionally, the UHPLC method enhances reproducibility in proteomic analysis due to its precise control over chromatographic conditions and consistent performance across different runs [7]. This protocol also reduces the time required for sample preparation and analysis compared to traditional methods, allowing for quicker turnaround and higher throughput.
Despite its advantages, the UHPLC-based protocol has some limitations. The protocol requires access to advanced UHPLC equipment, which may not be available in all research laboratories. Implementing this protocol necessitates a certain level of technical expertise in UHPLC and proteomic analysis, which may require specialized training for some laboratory personnel. Beyond muscle proteomics, this protocol can be adapted for use in other research areas that involve complex protein mixtures. For example, researchers can apply this protocol to nutritional studies, examining proteomic changes in muscle tissues in response to different dietary interventions and providing insights into the effects of nutrition on muscle health and function [8]. Additionally, the protocol can be used to evaluate the molecular effects of pharmacological agents on muscle tissues, aiding in the development of new drugs aimed at treating muscle-related diseases or enhancing muscle performance [9].
In summary, the UHPLC-based protocol for muscle proteomics offers significant improvements in resolution, sensitivity, reproducibility, and efficiency over traditional methodologies. While it does require specialized equipment and expertise, its advantages make it a powerful tool for advancing research in exercise physiology, muscle adaptation, and a wide range of other biomedical fields. This protocol not only enhances our understanding of molecular adaptations to exercise but also holds promise for broader applications in disease research, nutritional science, and drug development.
Materials and reagents
Biological materials
Human vastus lateralis muscle biopsy sample
Reagents
Povidone-iodine (Sigma-Aldrich, catalog number: PVP-I)
2% lidocaine with epinephrine (Hospira, catalog number: 0409-4279-16)
Glucose solution (Sigma-Aldrich, catalog number: G8769)
Physiological saline (Sigma-Aldrich, catalog number: S8776)
Liquid nitrogen (Air Products, catalog number: NI)
Isopentane (Sigma-Aldrich, catalog number: 277258)
Tris-HCl (Sigma-Aldrich, catalog number: T5941)
Triton X-100 (Sigma-Aldrich, catalog number: T8787)
Protease inhibitor cocktail (Sigma-Aldrich, catalog number: P2714)
BCA Protein Assay Kit (Thermo Fisher, catalog number: 23227)
Trichloroacetic acid (TCA) (Sigma-Aldrich, catalog number: T6399)
Acetone, HPLC grade (Sigma-Aldrich, catalog number: 34850)
Triethylammonium bicarbonate (TEAB) (Sigma-Aldrich, catalog number: T7408)
Trypsin (Promega, catalog number: V5111)
Dithiothreitol (DTT) (Sigma-Aldrich, catalog number: D0632)
Iodoacetamide (IAA) (Sigma-Aldrich, catalog number: I1149)
Formic acid (FA), HPLC grade (Sigma-Aldrich, catalog number: 56302)
Acetonitrile (ACN), HPLC grade (Sigma-Aldrich, catalog number: 34967)
Water, HPLC grade (Thermo Fisher, catalog number: W7-4)
EASY-nLC 1200 solvent A (0.1% formic acid in water) (Thermo Fisher, catalog number: LS1224)
EASY-nLC 1200 solvent B (0.1% formic acid in acetonitrile) (Thermo Fisher, catalog number: LS1225)
FAIMS Pro compensation voltage solution (Thermo Fisher, catalog number: FMSP 0003)
Solutions
PBS (see Recipes)
Triton lysis buffer (see Recipes)
LC-MS/MS mobile phase A (see Recipes)
LC-MS/MS mobile phase B (see Recipes)
Recipes
PBS
Reagent Final concentration Quantity or Volume NaCl 137 mM 8.01 g KCl 2.7 mM 0.2 g NaHPO 10 mM 1.42 g KHPO 1.8 mM 0.24 g Distilled H2O n/a Up to 1 L Triton lysis buffer
Reagent Final concentration Quantity or Volume Tris-HCl 50 mM 60.57 mg NaCl 150 mM 87.66 mg EDTA 1 mM 0.292 mg Triton X-100 1% 10 μL Protease inhibitor 1% 10 μL Distilled H2O n/a Up to 1 mL LC-MS/MS mobile phase A
Reagent Final concentration Quantity or Volume Formic acid 0.1% 1 mL (for 1 L) Acetonitrile 2% 20 mL (for 1 L) Water -- Up to 1 L LC-MS/MS mobile phase B
Reagent Final concentration Quantity or Volume Formic acid 0.1% 1 mL (for 1 L) Acetonitrile 90% 900 mL (for 1 L) Water -- Up to 1 L
Laboratory supplies
Disposable semi-automatic biopsy needle (Bard, catalog number: 1520-5010)
Pre-cooled tweezers (VWR, catalog number: 82027-530)
Aluminum foil (Reynolds, catalog number: 614)
Liquid nitrogen storage container (Thermo Fisher, catalog number: 11887163)
Cryogenic vials (Nalgene, catalog number: 5000-1020)
Mortar and pestle set (Bel-Art, catalog number: H37260-0000)
Microcentrifuge tubes (Eppendorf, catalog number: 0030120086)
1.5 mL microcentrifuge tubes (Eppendorf, catalog number: 022363204)
50 mL conical tubes (Falcon, catalog number: 352070)
1,000 μL pipette tips (Rainin, catalog number: RT-L1000F)
200 μL pipette tips (Rainin, catalog number: RT-L200F)
10 μL pipette tips (Rainin, catalog number: RT-L10F)
Sterile gloves (Medline, catalog number: MDS195285)
Sterile surgical drapes (3M, catalog number: 1010)
Sterile gauze pads (Dynarex, catalog number: 3344)
Surgical mask (Medline, catalog number: NON27378)
Medical adhesive tape (3M, catalog number: 1530-1)
Medical bandages (Johnson & Johnson, catalog number: 2015)
96-well plates (Thermo Fisher, catalog number: 260836)
Equipment
Ultrasound machine (GE Healthcare, model: LOGIQ E9)
-80 °C freezer (Thermo Fisher, model: ULT2186)
Centrifuge (Eppendorf, model: 5424)
Ultrasonic homogenizer (Qsonica, model: Q55)
Vortex mixer (VWR, model: 945302)
UHPLC system (Thermo Fisher, model: EASY-nLC 1200)
nanoACQUITY HPLC HHS T3 column (Waters, catalog number: 186003538)
Mass spectrometer (Thermo Fisher, model: Orbitrap Exploris 480)
FAIMS Pro Interface (Thermo Fisher, catalog number: FAIMS01-10000)
Water bath (Grant Instruments, model: JB Academy)
pH meter (Mettler Toledo, model: SevenCompact)
Magnetic stirrer (IKA, model: RCT basic)
Analytical balance (Sartorius, model: Entris II)
Refrigerated microcentrifuge (Eppendorf, model: 5418 R)
Electronic pipettes (Eppendorf, model: Xplorer plus)
Software and datasets
UniProt (https://www.uniprot.org/) (Access date, 2021/08)
Proteome Discoverer (v2.4.1.15, Thermo Scientific)
Procedure
文章信息
稿件历史记录
提交日期: Jun 19, 2024
接收日期: Oct 10, 2024
在线发布日期: Oct 31, 2024
出版日期: Dec 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Du, J., Hou, J., Yun, H. and Song, Y. (2024). Muscle Biopsy Sample Preparation and Proteomics Analysis Based on UHPLC-MS/MS. Bio-protocol 14(24): e5137. DOI: 10.21769/BioProtoc.5137.
分类
系统生物学 > 蛋白质组学 > 分泌蛋白质组
生物化学 > 蛋白质
细胞生物学 > 基于细胞的分析方法 > 蛋白质分泌
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link