- EN - English
- CN - 中文
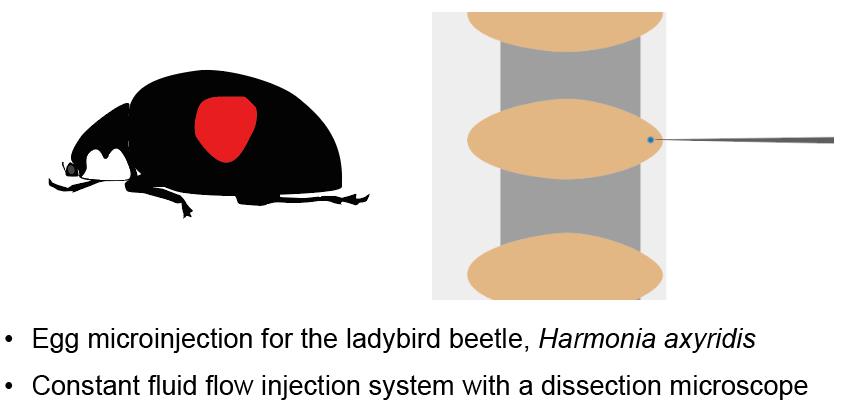
Egg Microinjection for the Ladybird Beetle Harmonia axyridis
异色瓢虫卵微注射方法
发布: 2024年12月20日第14卷第24期 DOI: 10.21769/BioProtoc.5136 浏览次数: 1861
评审: Alberto RissoneKai YuanAnonymous reviewer(s)
Abstract
In this paper, we present a detailed protocol for microinjecting DNA, RNA, or protein solutions into fertilized eggs of the multicolored Asian ladybird beetle, Harmonia axyridis, under a stereomicroscope equipped with an injection apparatus. H. axyridis is an emerging model organism for studying various biological fields, showing intraspecific polymorphisms exhibiting highly diverse color patterns on the elytra. Here, we describe how to rear ladybird beetles in a laboratory and obtain fertilized eggs for microinjection experiments. We also provide a constant fluid flow injection method, which enhances the efficiency of microinjection and improves throughput. Our step-by-step protocol is applicable to generating transgenic or genome-edited ladybird beetles, facilitating functional genetics in H. axyridis; the microinjection method should be applicable to other insect eggs.
Key features
• Egg microinjection for the multicolored Asian ladybird beetle Harmonia axyridis under a stereomicroscope with injection equipment.
• Detailed procedures for post-injection care, including rearing Harmonia axyridis.
• Step-by-step guide for the efficient collection of Harmonia axyridis eggs.
Keywords: Egg microinjection (卵微注射)Graphical overview
Background
Ladybird beetles belong to the order Coleoptera and encompass approximately 6,000 species worldwide, with around 190 species being described in Japan [1,2]. These beetles display diverse color patterns on their elytra, primarily black and red, which are not only species-specific but also display a high degree of diversity within species (intraspecific polymorphism) (reviewed in Ando and Niimi [3]). Of ladybird beetles, Harmonia axyridis has become an emerging model organism in diverse biological fields, such as color patterning [4,5], population genetics of intraspecific polymorphism [6,7], biological insecticides [8], and phenotypic plasticity [9]. H. axyridis possesses desirable characteristics as an experimental animal, such as the availability of an artificial diet, long-term storage of adults at low temperatures (up to 2 years and 8 months; personal observation), commercially available adults, high fecundity, easy access to fertilized eggs, and ease of sexing in adults based on morphology and at any stage by PCR-based techniques [10]. The ladybird beetle's lifecycle is relatively short—approximately 3 days for embryogenesis, around 11 days for larval development through three molts, 4.5 days for pupal development, and a lifespan of 2–3 months for adults (Figure 1). Furthermore, modern experimental techniques, including high-quality genome data [11,12], cryopreservation of the gonad [13], and numerous useful transgenic lines and mutant strains [14–16] including flightless mutants [17], and modern functional genomics, including RNAi, transgenics, and genome editing, have been developed for this species [4,14,15,18–20].
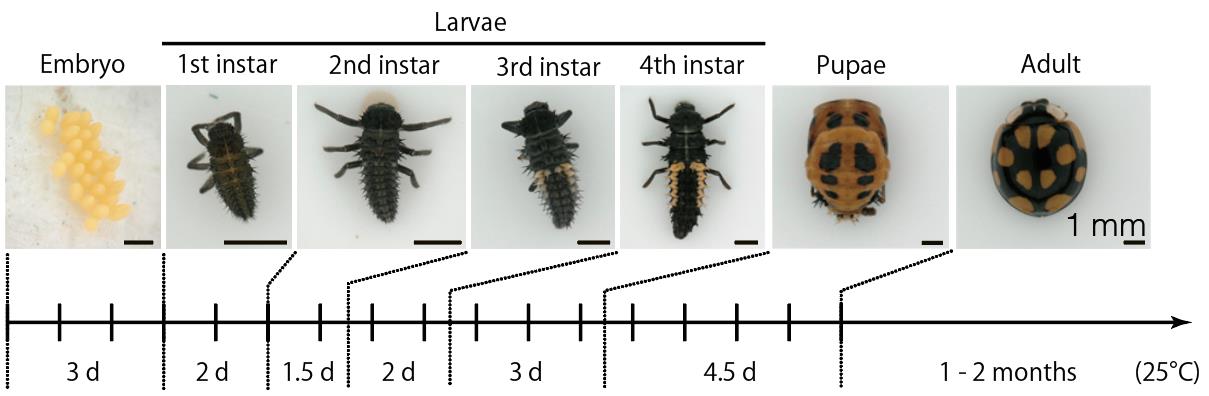
Figure 1. Life stages of Harmonia axyridis. The developmental period for each stage under laboratory conditions at 25 °C is indicated at the bottom. The period shown for adults represents the time during which they lay eggs after mating. It takes approximately one week after eclosion for adults to reach sexual maturity. Scale bar: 1 mm.
Fertilized egg microinjection is an essential approach for functional genomics including transgenesis and genome editing, being the most commonly used method in insects. While there are several methods for delivering reagents into eggs—including electroporation [21–23] and particle guns [24–26], which enable simultaneous treatment of multiple eggs—microinjection is the most widely used method for introducing nucleic acids, proteins, and chemicals into insect eggs. This method, however, has problems such as frequent clogging due to the backflow of egg contents, which prevents efficient injection. To overcome this problem, we developed an injection method using constant fluid flow (i.e., constant pressure during injection). This method not only prevents needle clogging but also contributes to increased injection throughput because it allows the delivery of the injection solution without egg-by-egg manual injection. However, this microinjection system is not able to control a precise injection volume, but the injection volume can be controlled by adjusting the time the needle is kept inside the egg. Our injection system is performed under a stereomicroscope with a micromanipulator and an injection needle holder, making the construction of an injection system more affordable than systems with compound microscopes. This paper describes the methodology of microinjection into the multicolored Asian ladybird beetle embryos using constant fluid flow injection with a stereomicroscope.
Materials and reagents
Biological materials
Harmonia axyridis (young-adult female and male beetles). Ladybird beetles are commercially available as biopesticides in Japan (Tentop, Agrisect, Japan) and possibly other countries. We used established lab culture lines once they had been collected in Aichi, Japan from the outside environment in the spring.
Pea aphid, Acyrthosiphon pisum (feeding source, important for high egg productivity)
Note: Live pea aphids are not commercially available. Pea aphids are found all over the world and can be self-fertilized. Aphids can be maintained on broad bean plants and are easily reared as they are asexual to ensure a steady food supply for the beetles.
Laboratory supplies
Micro-loading tip (Eppendorf, catalog number: 5242956003)
Paper towel (laid on the bottom of the rearing case)
Double-sided tape (Nitoms, catalog number: J0820 or equivalent)
Slide glass (Matsunami, catalog number: S1111)
Artist’s brush (00 number painting brush)
Glass capillary (NARISHIGE, catalog number: GD-1)
Equipment
FemtoJet 4i (Eppendorf, catalog number: 5252000021)
Dissection/stereomicroscope (Zeiss or equivalent)
Micromanipulator (Narishige, catalog number: MMO-202ND)
Micromanipulator stand (Narishige, catalog number: GJ-1)
Micromanipulator stand base (Narishige, catalog number: IP)
Needle holder (Narishige, catalog number: HI-7)
Micropipette puller (Sutter Instruments, catalog number: P-1000)
Box filament (FB245B, 2.5 mm × 4.5 mm)
Microcentrifuge (TOMY, model: MX-307)
Pipetman [GILSON, catalog numbers: F123602 (P1000), F123601 (P200), F123600 (P20), and F144801 (P2)]
Vortex mixer (LMS, model: VTX-3000L)
Insect breeding case (SPL Life Sciences, catalog number: SPL-310102)
Water container (see Figure 2; Umano Kagaku Youki Kabushiki Gaisya, Pla tsubo 20 mL) with a hole (3 mm diameter) on a lid
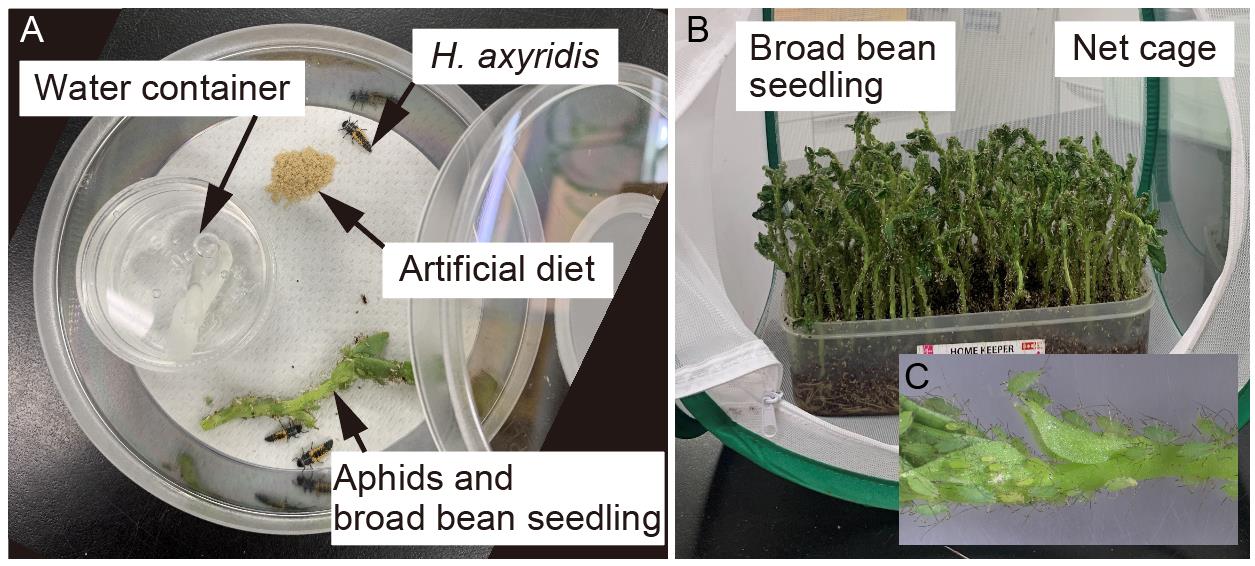
Figure 2. Post-injection care. (A) Overview of the H. axyridis rearing system. (B) Overview of the aphid rearing. Aphids are reared on the broad bean seedlings in a net cage. (C) Magnified view for aphids.Paper-based string for water supply (see Figure 2; Morioto Co., Ltd. 2 mm wide) cut at 4 cm long and placed in the water container
Hairdryer (Koizumi, catalog number: KHD-1430 or equivalent)
Clamp for holding hairdryer (Sibata Scientific Technology or equivalent)
Slide glass case (to keep premade egg holder slide clean)
Humid chamber (see Figure 3H)
Procedure
文章信息
稿件历史记录
提交日期: May 24, 2024
接收日期: Oct 10, 2024
在线发布日期: Oct 30, 2024
出版日期: Dec 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Nakamura, T., Matsuoka, Y. and Niimi, T. (2024). Egg Microinjection for the Ladybird Beetle Harmonia axyridis. Bio-protocol 14(24): e5136. DOI: 10.21769/BioProtoc.5136.
分类
发育生物学 > 基因组编辑
环境生物学
分子生物学 > DNA > 基因表达
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link