- EN - English
- CN - 中文
Mouse Model of Lipopolysaccharide (LPS)-Induced Pulpitis
脂多糖(LPS)诱导牙髓炎的小鼠模型
发布: 2025年01月20日第15卷第2期 DOI: 10.21769/BioProtoc.5128 浏览次数: 3332
评审: Komuraiah MyakalaShivaram SelvamMinal EngavaleRaghavendra Yelahanka Nagaraja
Abstract
Pulpitis is an important and prevalent disease within the oral cavity. Thus, animal models are necessary tools for basic research focused on pulpitis. Researchers worldwide often use dogs and miniature pigs to construct animal models of pulpitis. However, gene editing in miniature pigs is difficult, the surgical modeling process is complex, and tooth demineralization time is lengthy. Although some researchers have attempted to establish a mouse model of pulpitis, most models have involved direct exposure of dental pulp. However, the causes of pulpitis vary considerably among individuals, hindering effective research. In this study, we established a mouse model of pulpitis by accessing the pulp cavity, exposing the pulp to lipopolysaccharide (LPS), and then filling the tooth. One day after surgery, we observed many necrotic tissues and extensive inflammatory exudate, including neutrophils, around the coronal cavity preparation. Additionally, we noted many more neutrophils and a small amount of chronic inflammatory cell infiltrates at the junction between inflamed and normal tissue. These findings indicated that our model can be used to explore the early stage of pulpitis. Ten days after surgery, we observed vacuolar degeneration in some fibroblasts and proliferation in others at the distal end of the inflamed tissue. We also noted dilation and congestion of the pulp blood vessels. Therefore, our model can also be used to explore the middle and later stages of pulpitis. Thirty days after surgery, we observed necrosis in the coronal pulp cavity and upper half of the root pulp, indicating that our model can also be used to explore the end stage of pulpitis. This model is easy to establish, shows pulpitis progression in the dental pulp, exhibits a clear inflammatory phenotype, and can be readily combined with gene editing techniques. Accordingly, it is suitable for basic research focused on pulpitis and has substantial practical value.
Key features
• Lipopolysaccharide (LPS) can induce pulpitis in mice.
• The mouse model of LPS-induced pulpitis can be used in basic studies of pulpitis.
• After 1 day, the mouse model of LPS-induced pulpitis can demonstrate the main phenotypes of early-stage pulpitis.
• After 10 days, the mouse model of LPS-induced pulpitis can display the main phenotypes of middle and late stage pulpitis.
Keywords: Pulpitis (牙髓炎)Graphical overview
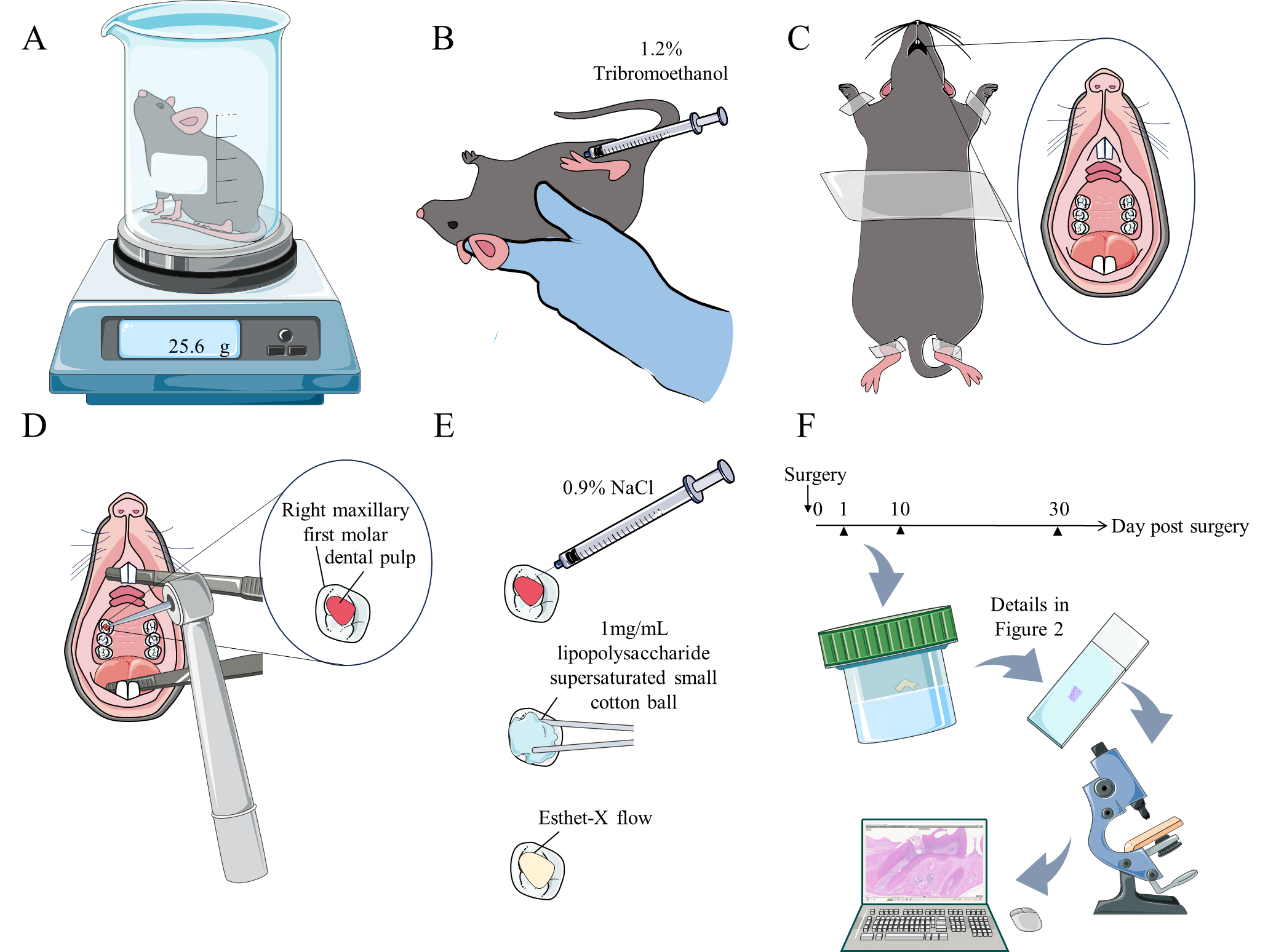
Figure 1. Graphical overview of the C57BL/6 mouse model of lipopolysaccharide (LPS)-induced pulpitis. A. Weigh the mouse. B. Anesthetize the mouse. C. Secure the mouse to the surgical pad and expose its oral cavity. D. Open the pulp chamber of the right maxillary first molar. E. Rinse the medullary foramen with 0.9% NaCl solution. Apply a small cotton ball saturated with 1 mg/mL LPS to the medullary foramen for 5 min, then cover the medullary foramen with Esthet-X flow and irradiate the site. F. Perform tissue decalcification and paraffin embedding (sample collection, decalcification, dehydration, wax embedding, and sectioning), followed by Histopathology staining, microscopy examination, image acquisition, and analysis.
Background
Although pulpitis is a common oral disease caused by anaerobic bacteria, its underlying mechanisms remain poorly understood. The construction of an animal model that simulates the processes involved in human pulpitis can aid in exploring the onset, progression, and outcomes of pulpitis. Thus far, pulpitis models have been constructed in animals such as mice, rats [1], ferrets, cats, dogs, miniature pigs, and monkeys [2]. However, because of their size, larger animals are more expensive and resource-intensive to maintain. Considering that experimental procedures in mice are relatively clear, gene editing is mainly conducted in these organisms, and the cost of mouse feed is low, there is a need to construct a mouse model of pulpitis.
Mouse molars are similar to human molars in many aspects, such as structure and cell types [3]; therefore, mouse models of pulpitis can be used to simulate the progression of human pulpitis. However, mouse molars are small, require skilled experimental techniques, and are difficult to manipulate; as such, mouse models of pulpitis have not been widely used. Most existing mouse models of pulpitis are induced by extended exposure of dental pulp after the pulp chamber has been opened [4–6]. This surgical method does not allow identification of the bacterial species causing pulpitis and is easily influenced by various biological factors, such as stimulant components in the feed and drink, or bacteria introduced into the oral cavity after surgery. In this study, we used lipopolysaccharide (LPS), an inflammatory factor often produced by anaerobic bacteria, as the pulpitis-inducing factor. After model induction, we capped the pulp to exclude interference from oral bacteria.
Materials and reagents
Biological materials
C57BL/6JCnc (B6J) mice (Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing)
Eight-week-old C57BL/6 mice, weighing 25–30 g, were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. and maintained in a pathogen-free environment within Capital Medical University Animal Experiment Center. All animal experiments were approved by the ethics committee of Capital Medical University (AEEI-2024-071). Mice were maintained on a 12:12 h light/dark cycle and given food and water ad libitum. C57BL/6 mice, among the most popular inbred mouse strains in many research laboratories worldwide, were used in the experiments described below.
Reagents
LPS-Pg (InvivoGen, catalog number: tIrl-pglps)
Endotoxin-free water (InvivoGen, catalog number: h2olal-1.5)
Esthet-X flow (DENTSPLY DeTrey GmbH, catalog number: 005-SZ2648021)
75% ethyl alcohol (ANNJET, catalog number: Q/371402AAJ008)
Tribromoethanol (Sigma-Aldrich, catalog number: 75-80-9)
NaCl (Hong Kong JiSiEnBei International Trade Co., Ltd., catalog number: JS0492-1KG)
Tissue specimen fixative (Servicebio, catalog number: G1101-15ML)
Xylene (Sinopharm Group Chemical Reagent Co., Ltd., catalog number: 10023418)
Absolute ethanol (Sinopharm Group Chemical Reagent Co., Ltd., catalog number: 100092683)
Ethylenediaminetetraacetic acid (EDTA) decalcification solution (Servicebio, catalog number: G1105)
Benzyl alcohol (Sinopharm Group Chemical Reagent Co., Ltd., catalog number: 30020618)
Environmentally friendly dewaxing and clearing solution (Servicebio, catalog number: G1128-1L)
10% paraformaldehyde fixative (neutral) (Servicebio, catalog number: G1101)
H&E staining kit (Servicebio, catalog number: G1003)
Neutral gum (Sinopharm Group Chemical Reagent Co., Ltd., catalog number: 10004160)
Citric acid antigen repair solution (pH 6.0) (Servicebio, catalog number: G1202)
EDTA antigen repair solution (pH 9.0) (Servicebio, catalog number: G1203)
EDTA antigen repair solution (pH 8.0) (Servicebio, catalog number: G1206)
PBS buffer (Servicebio, catalog number: G0002)
Tissue autofluorescence quenching agent (Servicebio, catalog number: G1221)
Bovine serum albumin (BSA) (Servicebio, catalog number: GC305010)
DAPI staining reagent (Servicebio, catalog number: G1012)
Antifade mounting medium (Servicebio, catalog number: G1401)
MPO primary antibody (Servicebio, catalog number: GB15224)
DSPP primary antibody (Servicebio, catalog number: sc-73632)
Cy3-labeled goat anti-mouse IgG (Servicebio, catalog number: GB21301)
Goldner staining solution suit (Servicebio, catalog number: G1064)
Glacial acetic acid (Sinopharm Group Chemical Reagent Co., Ltd., catalog number: 10000218)
Hydrochloric acid (Sinopharm Group Chemical Reagent Co., Ltd., catalog number: 10011018)
Solutions
1.2% tribromoethanol (see Recipes)
0.9% NaCl (see Recipes)
1 mg/mL LPS-Pg (see Recipes)
95% ethyl alcohol (see Recipes)
90% ethyl alcohol (see Recipes)
85% ethyl alcohol (see Recipes)
1% hydrochloric acid alcohol solution (see Recipes)
0.2% glacial acetic acid (see Recipes)
Recipes
1.2% tribromoethanol
Reagent Final concentration Amount Tribromoethanol (absolute) 1.2% 0.12 g H2O n/a 10 mL Total n/a 10 mL Weigh 0.12 g of tribromoethanol using an electronic scale with 0.001 g accuracy and dissolve it in 10 mL of distilled water; then, mix thoroughly with a vortex mixer.
0.9% NaCl
Reagent Final concentration Amount NaCl (absolute) 0.9% 0.9 g H2O n/a 100 mL Total n/a 100 mL Weigh 0.9 g of NaCl using an electronic scale with 0.1 g accuracy and dissolve it completely in 100 mL of distilled water.
1 mg/mL LPS-Pg
Reagent Final concentration Amount LPS-Pg (absolute) 1 mg/mL 1 mg Endotoxin-free water n/a 1 mL Total n/a 1 mL Aspirate 1 mL of endotoxin-free water with a 1 mL injection syringe and inject it into a glass medicine bottle containing 1 mg of LPS-Pg powder; mix these components to obtain a 1 mg/mL LPS-Pg solution.
Aliquot and label the LPS-Pg solution in sterile 0.1 mL tubes and freeze them at -20 °C until use.
Note: LPS-Pg solution at a concentration of 100 mg/mL is stable for >6 months if stored at -20 °C.
95% ethyl alcohol
Reagent Final concentration Amount Absolute ethanol 95% 95 mL Distilled water n/a 5 mL Total n/a 100 mL Using a 100 mL graduated cylinder, measure 95 mL of absolute ethanol and transfer it to a 100 mL beaker. Using a 5 mL graduated cylinder, measure 5 mL of distilled water and add it to the beaker containing ethanol. Add a magnetic stir bar to the beaker, place the beaker on a magnetic stirrer, and stir for 5 min.
90% ethyl alcohol
Reagent Final concentration Amount Absolute ethanol 90% 90 mL Distilled water n/a 10 mL Total n/a 100 mL Using a 100 mL graduated cylinder, measure 90 mL of absolute ethanol and transfer it to a 100 mL beaker. Using a 10 mL graduated cylinder, measure 10 mL of distilled water and add it to the beaker containing ethanol. Add a magnetic stir bar to the beaker, place the beaker on a magnetic stirrer, and stir for 5 min.
85% ethyl alcohol
Reagent Final concentration Amount Absolute ethanol 85% 85 mL Distilled water n/a 15 mL Total n/a 100 mL Using a 100 mL graduated cylinder, measure 85 mL of absolute ethanol and transfer it to a 100 mL beaker. Using a 25 mL graduated cylinder, measure 15 mL of distilled water and add it to the beaker containing ethanol. Add a magnetic stir bar to the beaker, place the beaker on a magnetic stirrer, and stir for 5 min.
1% hydrochloric acid alcohol solution
Reagent Final concentration Amount Hydrochloric acid 1% 1,000 μL Absolute alcohol n/a 99 mL Total n/a 100 mL Using a 100 mL graduated cylinder, measure 99 mL of absolute ethanol and transfer it to a 100 mL beaker. Using a 1,000 μL pipette, measure 1,000 μL of hydrochloric acid and add it to the beaker containing ethanol. Add a magnetic stir bar to the beaker, place the beaker on a magnetic stirrer, and stir for 30 s.
0.2% glacial acetic acid
Reagent Final concentration Amount Glacial acetic acid 0.2% 200 μL Distilled water n/a 99.8 mL Total n/a 100 mL Using a 200 μL pipette, measure 200 μL of glacial acetic acid and add it to a 100 mL volumetric flask. Add distilled water to the flask while stirring with a glass rod until the solution reaches a volume of 100 mL.
Laboratory supplies
Diamond bur (MANI, catalog number: TC-SS21F)
Dental file (Velbon, catalog number: K23120)
Operating scissors (Velbon, catalog number: J21030)
Ophthalmic forceps, straight (Velbon, catalog number: JD1050)
Ophthalmic forceps, curved (Velbon, catalog number: JD1060)
Dressing forceps (Velbon, catalog number: J42035)
Icebox (Biosharp, catalog number: BC032)
Icebox, silicone base (Biosharp, catalog number: BC034)
1 mL injection syringe (needle dimensions: 0.45 × 15 mm)
Pipette (10 μL, 200 μL, 1,000 μL) (Servicebio, catalog numbers: SPIP-10, SPIP-200, SPIP-1000)
Bagged tips (10 μL, 200 μL, 1,000 μL) (Servicebio, catalog numbers: P-10, P-200, P-1000)
0.1 mL centrifuge tubes (Thermo Fisher Scientific, catalog number: 4358297)
0.1 mL centrifuge tube rack (Servicebio, catalog number: WGH002)
Nitrile gloves (Servicebio, catalog number: GN1801M)
Stainless-steel lunch box (China Industry Union, catalog number: WGH0001)
Oral surgery kit (HENAN SHENG YUBEI EISAI Co., Ltd., catalog number: 20202171244)
Medical adhesive tape
Medical cotton balls: large and small
Permanent ink markers
Square medical sharps container
Medical waste garbage bags
Beaker
Doctor's scrubs
Embedding frame (Servicebio, catalog number: EF-1)
Ophthalmic scissors (Velbon, catalog number: JC2303)
Immunohistochemical pen (Servicebio, catalog number: G6100)
Graduated cylinder (5 mL, 10 mL, 25 mL, 100 mL)
Magnetic stirrer (Servicebio, catalog number: MS-150)
Magnetic stir bar (Servicebio, catalog number: WGA0023)
Volumetric flask (100 mL)
Equipment
Electronic scale with 0.1 g accuracy (LICHEN, catalog number: YP10001B)
Electronic scale with 0.001 g accuracy (Sartorius, catalog number: BCA224I-1OCN)
-20 °C freezer (MeiLing, catalog number: DW-YL450)
Autoclave (China Industry Union)
Head-mounted dental loupe (3.5×, black, 5 W headlight, China Industry Union)
Vortex mixer (Servicebio, catalog number: SMV-3500)
Portable dental treatment machine (Greeloy, catalog number: GU-P206S)
Timer
Dehydrator (DIAPATH, model: Donatello)
Embedding machine (Wuhan Junjie Electronics Co., Ltd., catalog number: JB-P5)
Freezing platform (Wuhan Junjie Electronics Co., Ltd., catalog number: JB-L5)
Constant temperature shaker (TIANJIN LEIBO TERRY EQUIPMENT Co., Ltd., catalog number: ZHPW-250)
Microtome (Shanghai Leica Instrument Co., Ltd., catalog number: RM2016)
Tissue spreader (Zhejiang Kehua Instrument Co., Ltd., catalog number: KD-P)
Oven (Tianjin Laibo Rui Instrument Equipment Co., Ltd., catalog number: GFL-230)
Adhesive slides (Servicebio, catalog number: G6012)
Cover glass (Citotest Labware Manufacturing Co., Ltd., catalog number: 10212432C)
Upright optical microscope (Nikon, model: NIKON ECLIPSE E100)
Imaging system (Nikon, model: NIKON DS-U3)
Decolorization shaker (Servicebio, catalog number: DS-2S100)
Microwave oven (Galanz, catalog number: P70D20TL-P4)
Scanner (3DHISTECH, model: Pannoramic MIDI)
Software and datasets
CaseViewer (Version: 2.4; Copyright © 2001-2020 3DHISTECH Ltd.; Build: 2.4.0.119028)
ImageJ 1.54f (Wayne Rasband and contributors; National Institutes of Health, USA)
Procedure
文章信息
稿件历史记录
提交日期: Apr 1, 2024
接收日期: Oct 5, 2024
在线发布日期: Oct 23, 2024
出版日期: Jan 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Shao, L., Chen, B. and Zheng, Y. (2025). Mouse Model of Lipopolysaccharide (LPS)-Induced Pulpitis. Bio-protocol 15(2): e5128. DOI: 10.21769/BioProtoc.5128.
分类
生物科学 > 生物技术
医学 > 发炎
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









