- EN - English
- CN - 中文
Development of a Rapid Epstein–Barr Virus Detection System Based on Recombinase Polymerase Amplification and a Lateral Flow Assay
基于重组酶聚合酶扩增和侧向流动检测的快速EB病毒检测系统开发
发布: 2024年12月05日第14卷第23期 DOI: 10.21769/BioProtoc.5122 浏览次数: 1613
评审: Emilia KrypotouChhuttan L MeenaJibin Sadasivan
Abstract
The quality of cellular products used in biological research can impact the accuracy of results. Epstein–Barr virus (EBV) is a latent virus that spreads extensively worldwide, and cell lines used in experiments may carry EBV and pose an infection risk. The presence of EBV in a single cell line can contaminate other cell lines used in the same laboratory, affecting experimental results. Existing tests to detect EBV can be divided into three categories: nucleic acid assays, serological assays, and in situ hybridization assays. However, most methods are time-consuming, expensive, and not conducive to high-volume clinical screening. Therefore, a simple system that allows for the rapid detection of EBV in multiple contexts, including both cell culture and tissue samples, remains necessary. In our research, we developed EBV detection systems: (1) a polymerase chain reaction (PCR)-based detection system, (2) a recombinase polymerase amplification (RPA)-based detection system, and (3) a combined RPA-lateral flow assay (LFA) detection system. The minimum EBV detection limits were 1 × 103 copy numbers for the RPA-based and RPA-LFA systems and 1 × 104 copy numbers for the PCR-based system. Both the PCR and RPA detection systems were applied to 192 cell lines, and the results were consistent with those of the assays specified in industry standards. A total of 10 EBV-positive cell lines were identified. The combined RPA-LFA system is simple to operate, allowing for rapid result visualization. This system can be implemented in laboratories and cell banks as part of a daily quality control strategy to ensure cell quality and experimental safety and may represent a potential new technique for the rapid detection of EBV in clinical samples.
Key features
• Establishes RPA and RPA-LFA detection systems for EBV.
• The RPA-LFA detection system can visualize the results in as short as 15 min.
• The specificity of the RPA and RPA-LFA assay systems for the detection of EBV is validated.
• The minimum EBV detection limit of the RPA and RPA-LFA systems is 1 × 103 copies.
Keywords: RPA (RPA)Graphical overview
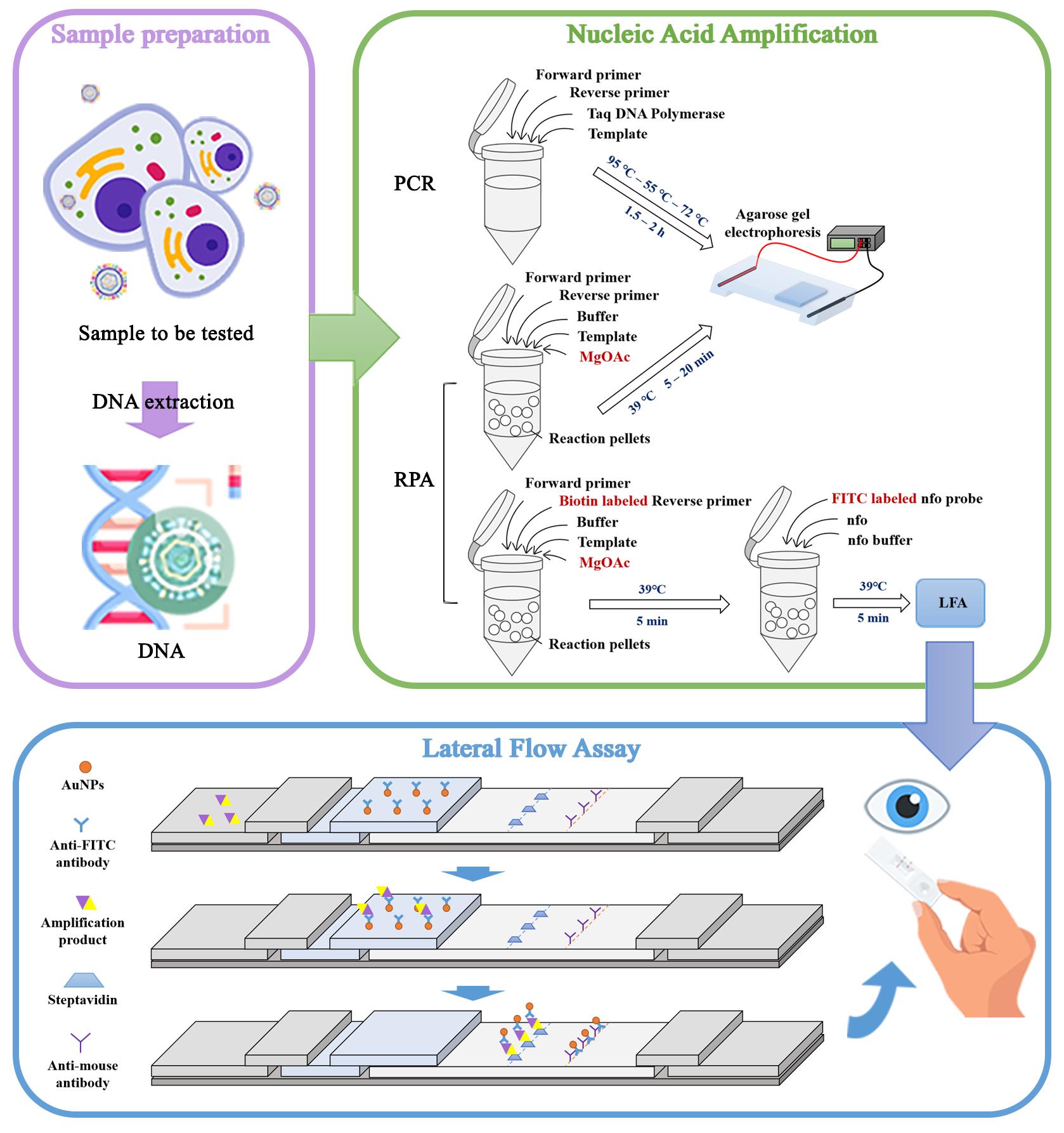
Pattern of Epstein–Barr virus (EBV) detection by the PCR, recombinase polymerase amplification (RPA), and combined RPA-lateral flow assay (LFA) systems. Reaction pellets are small particles that contain key reagents. These pellets usually contain the core components needed for the RPA reaction (recombinase, recombinase loading factor, and single-stranded binding protein). At the start of the reaction, reaction pellets dissolve these components to initiate the amplification reaction.
Background
As biological research continues to advance, cells are increasingly at risk of being misidentified or contaminated with other cell types or exogenous factors [1,2] including viruses; such contamination can be difficult to detect and remediate.
Epstein–Barr virus (EBV) is a member of the human herpesvirus family. EBV displays prolonged latency in lymphocytes, interfering with immune functions and potentially inducing cell proliferation and transformation. EBV infection involves many organ systems and is often misdiagnosed or underdiagnosed. Multiple reports in the literature have described the detection of EBV infection in various cell types maintained in cell culture banks [3,4]. These infections can be attributed to the presence of an existing EBV infection during the initial process of cell line establishment, EBV contamination of culturing materials, or improper manipulation by the experimental staff.
The most widely used of the existing EBV assays is PCR, which is a well-established and reliable molecular method. Quantitative PCR (qPCR) can be used to monitor disease progression or therapeutic efficacy by detecting changes in EBV load in the blood [5]. Iso-thermal amplification techniques, such as recombinant enzyme-mediated isothermal amplification and loop-mediated isothermal amplification (LAMP), are easy to perform, have limited equipment requirements, return rapid responses, and display high sensitivity. However, designing primers for use in the LAMP technique is complicated and can be difficult to replicate. In situ hybridization assay to detect Epstein–Barr early RNA (EBER) is the current gold standard for detecting EBV infection in clinic. However, this technique can only be applied to tissue samples and is generally limited to the clinical diagnosis of EBV-related diseases, such as cancer and lymphoma.
Recombinase polymerase amplification (RPA) is a highly sensitive and selective isothermal amplification technique that can be performed at 39 °C and can be used to amplify a large number of samples in a short period of time. RPA, first introduced in 2006 by Niall Armes of ASM Scientific Ltd., UK [6], relies on modified homologous recombination mechanisms. RPA reagents are commercially available, and the basic RPA reaction kit can be augmented by additional commercially available kits that use different probes that can be cleaved by different enzymes: exo (exonuclease III), fpg (formamidopyrimidine DNA Glycosylase), and nfo (endonuclease IV) [7]. The exo and fpg probes are typically used for real-time detection, whereas the nfo probe is typically used for detection systems that use lateral flow dipsticks.
In this study, we combined RPA technology with a lateral flow assay (LFA) to develop an RPA-LFA detection system for application to the rapid and bulk screening of EBV contamination in cell lines stored by cell banks and to conduct regular and daily inspection of cell lines used in biological experiments, ensuring the quality of cell lines and the safety of experimental personnel. This system also demonstrates high potential for clinical adaptation to improve EBV detection in blood and tissue samples.
Materials and reagents
Biological materials
B95-8 cell line (China Center for Type Culture Collection, GDC0015)
HeLa cell line (China Center for Type Culture Collection, GDC0009)
Epstein–Barr virus (China Center for Type Culture Collection, GDV132)
Reagents
Trypsin (AMEKO, catalog number: RC01145)
Ethanol (National Pharmaceutical Group Chemical Reagent Co., Ltd., catalog number: 10009228)
PBS (Gibco, catalog number: 70013032)
TIANamp Genomic DNA Kit (TIANGEN, catalog number: DP304)
E.Z.N.A Viral DNA Kit (OMEGA, catalog number: D3892)
Takara 10× loading buffer (Takara, catalog number: AA0151)
DL2,000 DNA marker (Takara, catalog number: 3427A)
TS-GelRed nucleic acid dye (TSINGKE, catalog number: TSJ003)
Agarose (TSINGKE, catalog number: TSJ001)
RNase-free water (Takara, catalog number: takara.9012)
Twist Amp Basic kit (TwistDX, catalog number: 111-035-003)
Thermo Scientific endonuclease IV(Nfo) and its buffer (FastDigest, catalog number: EN0591)
Test strip dilution (Aoke Botai Biotech)
Primer and probe:
FP: 5'-CTTGGAGACAGGCTTAACCAGACTCA-3'
RP: 5'-CCATGGCTGCACCGATGAAAGTTAT-3'
LFA-RP: 5'-[BIOTIN]CCATGGCTGCACCGATGAAAGTTAT-3'
LFA-Probe: 5'-[FITC]TGCCGGCCCCTCGAGATTCTGACCGGGGACC[THF]CTGGTTGCTCTGTTG[C3-Spacer]-3'
Laboratory supplies
Cell culture flask (NEST, catalog number: 707001)
Serological pipettes (NEST, catalog number: 327001)
Pipette tips (Biosharp, catalog numbers: BS-10-T, BS-200-T, BS-1000-T)
Micro-centrifuge tube (Biosharp, catalog number: BS-15-M)
Axygen PCR tubes (AXYGEN, catalog number: Axygen- PCR-05-A)
Lateral flow stick (Suzhou Xianda Gene Technology, catalog number: TS101)
Equipment
Biological safety cabinet (Esco, catalog number: A2AC2-S)
Refrigerator [CHANGHONG MEILING, catalog number: MCF(L)-398LDWEP]
Thermostatic water bath (Dichbio, catalog number: JB Academy)
PCR automatic serialization analyzer (SensoQuest, catalog number: Labcycler 48)
Low-speed centrifuge (DLAB, catalog number: D1008E, D1008)
Small high-speed freezer-type centrifuge (Eppendorf, model: 5420)
CO2 Incubator (Memmert, catalog number: INC)
Ultra-micro UV spectrophotometer (Thermo Fisher Scientific, model: NanoDrop2000)
Micropipettes 0.1–2.5 µL, 2–20 µL, 20–200 µL (Eppendorf, catalog number: 3123000217, 3123000233, 3123000250)
Electrophoresis apparatus (Beijing Liuyi Biotechnology Co., Ltd., catalog number: 112-0630)
Gel imaging system (Bio-Rad, catalog number: ChemiDoc XRS+)
Vortex mixer (Kylin-bell, catalog number: VORTEX-6)
Software and datasets
DNAMAN (version 8.0)
National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/)
Procedure
文章信息
稿件历史记录
提交日期: Aug 1, 2024
接收日期: Oct 4, 2024
在线发布日期: Oct 16, 2024
出版日期: Dec 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Sun, Y., Tang, D., Li, N., Wang, Y., Yang, M. and Shen, C. (2024). Development of a Rapid Epstein–Barr Virus Detection System Based on Recombinase Polymerase Amplification and a Lateral Flow Assay. Bio-protocol 14(23): e5122. DOI: 10.21769/BioProtoc.5122.
分类
微生物学 > 病原体检测 > PCR
分子生物学 > DNA > PCR
医学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link