- EN - English
- CN - 中文
A Simple, Rapid, and Cost-Effective Method for Assessing Carbohydrate Partitioning in Microalgae and Arabidopsis thaliana
一种简便、快速且经济的方法评估微藻和拟南芥的碳水化合物分配
(§ Technical contact) 发布: 2024年12月05日第14卷第23期 DOI: 10.21769/BioProtoc.5121 浏览次数: 2302
评审: Abhilash PadavannilWalmik Karbhari GaikwadJoyce Chiu
Abstract
Carbohydrates serve crucial functions in most living cells, encompassing structural and metabolic roles. Within the realms of plant and algal biology, carbohydrate biosynthesis and partitioning play pivotal roles in growth, development, stress physiology, and various practical applications. These applications span diverse fields, including the food and feed industry, bioenergetics (biofuels), and environmental management. However, existing methods for carbohydrate determination tend to be costly and time-intensive. In response to that, we propose a novel approach to assess carbohydrate partitioning from small samples. This method leverages the differential solubility of various fractions, including soluble sugars, starch, and structural polymers (such as cellulose). After fractionation, a straightforward spectrophotometric analysis allows for the quantification of sugars.
Key features
• We developed a cost-effective method to assess carbohydrate distribution in small samples based on differential solubility and spectrophotometry.
• Efficient carbohydrate partitioning methods reduce time and effort, especially for large sample sets.
• Cost-effective carbohydrate analysis method reduces expenses, promotes accessibility, and encourages adoption in research and quality control.
• Analysis of small samples from microalgal and A. thaliana seedlings has wide applicability in scientific and technological research.
Keywords: Sugars (糖类)Graphical overview
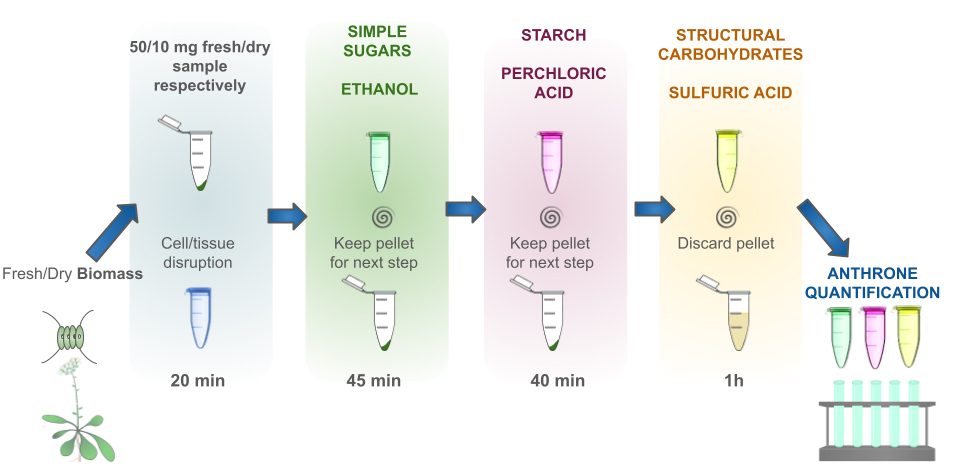
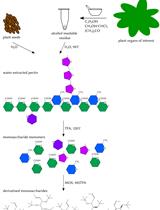
Description of the complete process. A total of 10 mg of dry sample or an equivalent quantity of fresh biomass is sufficient to carry out the extraction process. The initial step involves disruption of the samples through freezing and thawing. This is followed by the sequential extraction of simple sugars through an alcoholic extraction, which is then subjected to centrifugation to retain the supernatant. The resulting pellet is retained for starch extraction/hydrolysis by cold perchloric acid. The resulting insoluble polysaccharides from this fraction are extracted/hydrolyzed by hot sulfuric acid. Finally, sugars are determined by the anthrone method in every fraction collected, which corresponds to sugars, starch, and structural carbohydrates. The basic principles and methods for sugar extraction with ethanol, starch extraction/hydrolysis with perchloric acid, and polysaccharides hydrolysis with sulfuric acid have been described before [1,2].
Background
Carbohydrates play essential roles in most living cells, serving structural and metabolic functions. In algae and plants, soluble sugars are crucial for various metabolic processes, including osmotic regulation, energy translocation, and signal transduction. When carbon assimilation exceeds the demand for growth and maintenance, the excess is converted into starch. Moreover, starch accumulates within cells during unfavorable environmental conditions, allowing algae and plants to withstand severe challenges. Polysaccharides, in conjunction with proteins, are vital components of cell walls, influencing their conformation, resistance, and stability. While microalgae cell walls exhibit extensive diversity, they may contain cellulose, pectin, agar, or alginate [3]. Conversely, in the model plant A. thaliana, the primary cell wall primarily consists of pectin, hemicellulose, and cellulose [4].
Several techniques are currently available for the quantitative identification and determination of carbohydrates, namely chromatography methods (such as HPLC and GC–MS), enzymatic assays, colorimetric methods, and physical measurements based on a specific gravity or refractive index [5]. While these methods are highly accurate, they often require expensive equipment and high-quality reagents and can be time-consuming. Additionally, methods for determining multiple carbohydrates from the same sample remain scarce.
To address this gap, we have developed an easy, rapid, and cost-effective method for assessing carbohydrate partitioning in microalgae and plants. This approach allows high-throughput data acquisition. A modified protocol for smaller sample sizes and reduced reaction volumes is provided in the Supplementary information.
Materials and reagents
Biological materials
Chlamydomonas reinhardtii represents a valuable research organism with a sequenced genome, simple life cycle, and ability to grow in multiple metabolic states. It is a model organism for studies in areas like flagella structure, chloroplast biogenesis, photosynthesis, and cell–cell recognition. Its cell wall is composed of glycoproteins and polysaccharides in a multi-layered structure. Strain CC125 represents a popular wild-type (WT) alga for the species; strain cw15 [6] is a cell wall–deficient mutant that facilitates genetic manipulation and cellular process studies; and sta6 is a mutant strain deficient in starch accumulation, which is very useful for studies on carbon partitioning and lipid accumulation [7].
Scenedesmus obliquus and Chlorella sorokiniana are thoroughly used for biotechnological applications such as the production of biofuels and food/feed ingredients and wastewater treatment. They show very robust and prolific growth in cultivation facilities. In contrast to C. reinhardtii, they currently offer limited possibilities for genetic modification and reduced availability of mutant strains. While the S. obliquus cell wall comprises a resistant outer layer of algaenan and an inner layer of cellulosic material, the C. sorokiniana cell wall presents a thin trilaminate layer of hemicellulose and a glucosamine-based residue and produces mucilage [8].
A. thaliana, a model organism in plant biology, boasts features like a small genome, rapid life cycle, and easy genetic modification. Its cell wall, composed of cellulose, hemicellulose, and pectin, provides strength, flexibility, and porosity. Some cells even develop a secondary cell wall with lignin for rigidity. Structural proteins, enzymes, and glycoproteins contribute to cell signaling and growth. Remodeling occurs during growth and in response to environmental stress [9].
All of these organisms produce starch as a carbon and energy reserve, and sugars with different physiological roles.
Organisms' cultivation conditions
The microalgal strains C. reinhardtii CC125 (WT), cw15 (cell wall–deficient), and sta6 (starchless mutant) were kindly provided by C. Benning (Michigan State University), and strains C. sorokiniana RP and S. obliquus C1S were isolated [10] and characterized [11] by our research group. These algal strains belong to the INBIOTEC culture collection.
The microalgal strains were cultivated and induced for carbohydrate accumulation as previously described in Bader et al. [12] under nitrogen-deficient conditions.
The A. thaliana used in this study was a wild type of Columbia (Col-O) stored in our laboratory. Seeds were sown in pots and grown under 16/8 h light/dark (light 120 μmol photons m-2·s-1) at 23 °C in a growth room. Fourteen-day-old A. thaliana plants were treated with 150 mM NaCl for 14 days before leaves were collected for carbohydrate analysis.
Reagents
Distilled water (dH2O)
Double-distilled water (ddH2O) for anthrone solution
Ethanol 96% (C2H6O) (e.g., Alco Protect, Porta, Argentina)
Perchloric acid 60% (HClO4) (Fluka, catalog number: 77232)
Sulfuric acid 96%–98% (H2SO4) (Biopack, Brand, catalog number: 0303A0036)
Glucose analytical grade (C6H12O6) (Glc) (Sigma-Aldrich, catalog number: G5767)
Anthrone (C14H10O) [Fluka, catalog number: A 866 (0740)]
Thiourea (CH4N2S) (Sigma-Aldrich, catalog number: T7875-500G)
Note: Both sulfuric acid and perchloric acid are hazardous to touch and inhalation; therefore, it is necessary to work with caution and use personal protective equipment (gloves, lab coat, closed-toe shoes, and safety goggles).
Solutions
For carbohydrate quantification, the anthrone method was used, which has been widely validated and allows for the quantification of a broad range of carbohydrates, hexoses, and aldopentoses [13]. In the presence of concentrated sulfuric acid, carbohydrates are dehydrated into furfurals (or hydroxymethylfurfurals), which condense with anthrone (10-keto-9,10-dihydroxyanthracene) (Figure 1) to produce a blue-green complex. The intensity of the color is quantified by absorbance at 620 nm.
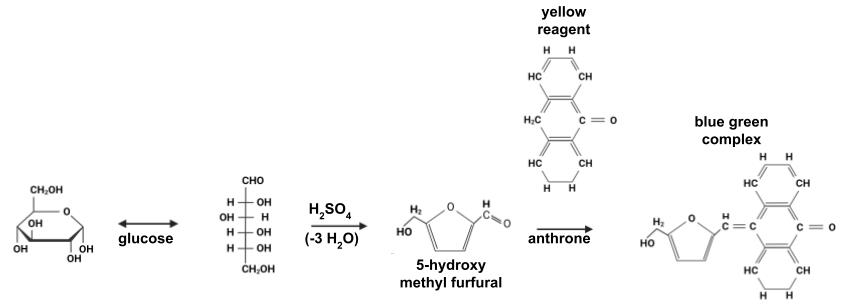
Figure 1. Reaction of the anthrone assay. Modified from Gerwig [14]. The extinction coefficient of the complex between 5-hydroxy methyl furfural and anthrone at 620 nm is 76,000 M-1·cm-1.
67% sulfuric acid solution (see Recipes)
Anthrone solution (72% sulfuric acid final concentration) (see Recipes)
10 mM glucose solution (see Recipes)
Recipes
67% sulfuric acid solution (10 mL)
Conduct the process under a fume hood and using personal protective equipment (gloves, lab coat, closed-toe shoes, and safety goggles). In a glass container, pour 3.3 mL of ddH2O. Place the container on ice and then slowly add 6.7 mL of sulfuric acid to prevent acid splashes upon contact with water.
Anthrone solution (100 mL)
Conduct the process under a fume hood and using personal protective equipment (gloves, lab coat, closed-toe shoes, and safety goggles). In a glass container, pour 28 mL of ddH2O. Place the container on ice and then slowly add 35 mL of sulfuric acid to prevent acid splashes upon contact with water, while stirring with a magnetic stir bar. Add 50 mg of anthrone and 1 g of thiourea. Complete with 37 mL of sulfuric acid. Leave to stir for 16 h in darkness.
10 mM Glc solution (10 mL)
Prepare by adding 18 mg of Glc to 10 mL of ddH2O.
Equipment
General lab supplies: microtubes, pipettes, tips, tube racks, etc.
Block heater (Thermolyne, model: Type 17600 Dri-Bath)
Bench-top vortex (Labnet, model: VX100)
Benchtop centrifuge for 2 mL tubes at room temperature and 4 °C (Thermo Scientific, model: Sorvall Legend Micro 21R)
Fume Hood (Esco, model: EFA-4UDRVW-8)
5 mL glass test tubes
Analytical balance (Sartorius, model: Entris2241-1S)
UV/VIS spectrometer (Shimadzu Corp., serial number A114548, 05789)
1 cm Pathlength quartz cuvettes; disposable or glass cuvettes can also be used
Gas mask (3M, model: 6899B, filters 3M 6003) for measuring samples with anthrone
Thermostatic water bath for laboratory (Daglef Patz, model: Hor, 18.3867)
Magnetic stirring bar and magnetic stirrer (Thermolyne, model: Nuova II)
Procedure
文章信息
稿件历史记录
提交日期: Jun 19, 2024
接收日期: Sep 26, 2024
在线发布日期: Oct 16, 2024
出版日期: Dec 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Bader, A. N., Rizza, L. S., De Marco, M. A., Lando, A. P., Martínez-Noël, G. M. A., Consolo, V. F. and Curatti, L. (2024). A Simple, Rapid, and Cost-Effective Method for Assessing Carbohydrate Partitioning in Microalgae and Arabidopsis thaliana. Bio-protocol 14(23): e5121. DOI: 10.21769/BioProtoc.5121.
分类
微生物学 > 微生物生物化学 > 糖类
植物科学 > 植物生物化学 > 糖类
生物化学 > 糖类
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link