- EN - English
- CN - 中文
A Protocol for co-Injecting Cells with Pulverized Fibers for Improved Cell Survival and Engraftment
细胞与粉碎纤维共注射提高细胞存活率和移植效果的实验方案
(*contributed equally to this work) 发布: 2024年11月20日第14卷第22期 DOI: 10.21769/BioProtoc.5117 浏览次数: 1407
评审: Pilar Villacampa AlcubierreShivaram SelvamAnonymous reviewer(s)
Abstract
Adipose tissue is crucial for medical applications such as tissue reconstruction, cosmetic procedures, and correcting soft tissue deformities. Significant advances in the use of adipose tissue have been achieved through Coleman’s studies in fat grafting, which gained widespread acceptance due to its effectiveness and safety. Despite its benefits, adipose tissue grafting faces several limitations, including high absorption rates due to insufficient support or anchorage, replacement by fibrous tissue, migration from the intended site, and loss of the initial desired morphology post-administration. To counteract these constraints, there is a need for improved grafting techniques that enhance the predictability and consistency of outcomes. Biomaterials are extensively used in tissue engineering to support cell adhesion, proliferation, and growth. Both natural and synthetic materials have shown promise in creating suitable microenvironments for adipose tissue regeneration. PLGA, a synthetic copolymer, is particularly notable for its biocompatibility, biodegradability, and tunable mechanical properties. Here, we describe a protocol using milled electrospun poly(lactic-co-glycolic acid) (PLGA) fibers combined with lipoaspirated tissue to create a fibrous slurry for injection. By pulverizing PLGA fiber mats to create fiber fragments with increased pore size and porosity, we can influence key cellular responses and enhance the success of adipose tissue–grafting procedures. This approach improves anchorage and support for adipocytes, thereby increasing cell viability. This method aims to enhance vascularity, perfusion, and volume retention in adipose tissue grafts, which addresses many of the limitations of current approaches to adipose tissue grafting and holds promise for more consistent and successful outcomes.
Key features
• Adipose tissue for tissue reconstruction.
• Need for improved engraftment and volume retention.
• Pulverized PLGA fiber mats to create a fibrous "slurry" that allows injection.
• PLGA fibers co-injected with lipoaspirated tissue.
• Improved adipose engraftment outcomes (e.g., perfusion, vascularity, and retention of graft volume).
Keywords: Pulverized PLGA fibers (粉碎PLGA纤维)Graphical overview
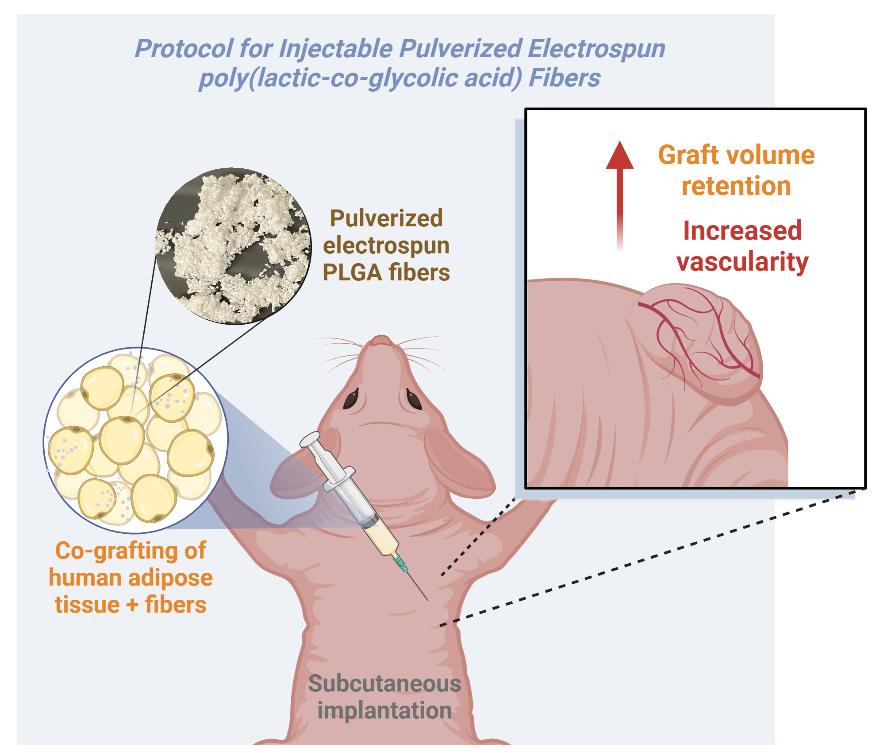
Overview of the subcutaneous implantation of human adipose tissue and pulverized electrospun poly(lactic-co-glycolic acid) (PLGA) fibers in a mouse model
Background
Adipose tissue plays a crucial role in numerous medical applications, such as tissue reconstruction, cosmetic procedures, and the correction of soft tissue deformities. The first use of autologous adipose tissue in humans was described in 1889 by Van der Meulen [1]. While many subsequent studies have explored this technique to correct various defects, a significant advancement in fat transplantation occurred with the publication of Coleman’s studies in fat grafting [2]. The Coleman technique, which utilizes autologous adipose tissue, has gained widespread acceptance due to its effectiveness and safety [3]. This method involves harvesting adipose tissue via liposuction, processing it, and reinjecting it into various tissue depths. Studies have demonstrated its efficacy in yielding viable adipocytes and sustaining optimal cellular function within fat grafts, making it a valuable method for soft-tissue augmentation and tissue repair [4].
The use of adipose tissue has led to numerous advancements in the use of autologous fat grafts not only in aesthetic treatments but also in various medical specialties such as therapies for breast cancer [2,5]. The regenerative potential of adipose tissue is attributed to the presence of stem cells, which makes it an ideal filler due to its availability, low donor-site morbidity, cost-effectiveness, and biocompatibility [6]. Adipose-derived stromal cells can secrete various growth factors, such as VEGF, HGF, and TGF-β, which play a crucial role in tissue remodeling. These growth factors influence the differentiation of stem cells and promote angiogenesis, thereby enhancing the efficacy of adipose tissue in tissue engineering approaches [7]. Moreover, after transplantation, ischemic adipocytes attract macrophages, initiating revascularization through neoangiogenesis, suggesting thus that the foreign body response is associated with increased vascularization [6,8].
However, existing autologous adipose engraftment methods have several limitations that impact the success and consistency of clinical outcomes. In clinical practice, a major issue with adipose tissue autotransplantation is the absorption rate over time, which ranges from 25% to 70% of the total implanted volume [2,6,9]. In 1987, the American Society of Plastic and Reconstructive Surgeons reported that only 30% of the injected autologous fat was expected to survive for one year [9]. Additionally, it was observed that the transplanted tissue often became filled with connective tissue, indicating the death of the adipose tissue followed by its replacement with fibrous tissue or newly formed metaplastic fat [9]. In contrast, Peer proposed the cellular survival theory, which suggests that the final volume after an adipose tissue transplant depends on the number of living adipocytes at the time of transplantation [9,10]. These challenges underscore the need for better grafting techniques to improve the predictability and consistency of adipose grafting results.
Biomaterials are extensively utilized in tissue engineering for their capability to support cell adhesion, proliferation, and growth during the development of new tissues. For instance, porous scaffolds and hydrogels, both natural and synthetic, have shown promise in improving adipose tissue grafting procedures. These materials have been found to enhance cell viability, promote vascularization, and support the growth of adipocytes derived from progenitor cells. Collagen-based scaffolds, hyaluronan-heparin-collagen hydrogels, fibrinogen hydrogels, and decellularized extracellular-matrix scaffolds are among the natural materials that have demonstrated potential in creating a suitable microenvironment for adipose tissue regeneration [11–13]. On the other hand, synthetic materials such as poly(N-isopropylacrylamide) (PNIPAm), polyglycolic acid (PGA), polymeric nanocomposites, and poly(lactic-co-glycolic acid) (PLGA) have shown promise in tissue regeneration, contributing to adipose tissue grafting [14,15].
In this context, we propose a protocol to improve grafting outcomes using milled/pulverized electrospun PLGA fibers combined with lipoaspirated tissue to create a fibrous slurry for injection into recipient tissue. PLGA is a copolymer widely used in tissue engineering due to its excellent biocompatibility, biodegradability, and mechanical properties. PLGA degrades into lactic acid and glycolic acid, which are naturally metabolized by the body, minimizing its toxicity [16]. Additionally, the mechanical properties (e.g., degradation rate) can be adjustable by altering the ratio of lactic acid to glycolic acid monomers. Higher lactic acid content results in slower degradation, while higher glycolic acid content leads to faster degradation. The ability to control the degradation rate by adjusting the ratio is a unique feature of PLGA that sets it apart from other commonly used biodegradable polymers, such as PGA or polylactic acid (PLA) [17,18]. While PLGA can be easily electrospun into microscale fibers for scaffolding in cell culture, these fibers must be processed into small pieces to make them suitable for injection. Therefore, we pulverize the electrospun fiber mats using a mini mill to create small fibrous clusters with increased pore size and porosity, which are critical for influencing key cellular responses.
We present a detailed protocol for using milled electrospun PLGA fibers co-injected with adipose tissue, aimed at enhancing vascularity, perfusion, and graft volume retention. This approach offers the potential to improve the consistency and success of adipose grafting techniques, addressing the limitations of current methods.
Materials and reagents
Reagents
Polylactide-co-glycolide acid (PLGA) 82:18 lactide:glycolide (Corbion Purac, catalog number: PLG8218)
Hexafluoroisopropanol (Oakwood Chemical, catalog number: 3409)
0.9% sodium chloride solution (saline) (Henry Shein, catalog number: 1047098)
Ethanol, 200 Proof (100%) (Decon Labs, catalog number: 2701)
Betadine (Henry Schein, catalog number: 67618–150-09)
Isopropyl alcohol (Fisher Scientific, catalog number: A416P-4)
Isoflurane (Primal Health Care, catalog number: NDC 66794–017-10)
Artificial tear ointment (Henry Schein, catalog number: 1338333)
Nairing cream (Nair, catalog number: 339823)
Solutions
70% ethanol solution (see Recipes)
Recipes
70% ethanol solution
Mix 35 mL of 100% ethanol with 15 mL of sterile water, to create 50 mL of 70% ethanol solution.
Laboratory supplies
0.5 mm sieve (IKA, model: 2939000)
0.25 mm sieve (IKA, model: 2938900)
2 mL Eppendorf Tubes (VWR, catalog number: 87003-298)
50 mL Falcon tubes (VWR, catalog number: 89039-660)
Alcohol swabs (Henry Shein, catalog number: HS1007)
Cotton tip applicator (McKesson, Q-Tip, catalog number: 785468)
Liquid glue (3M Vetbond, catalog number: B07Q39FL9M)
6–0, C-22 black braided silk sutures (Henry Schein, catalog number: 101–2636)
Surgical tape (3M, catalog number: 1527–0)
18-gauge needle (Central Infusion Alliance, catalog number: BD 305196)
3 mL syringe (Fisherbrand, catalog number: 14–955-457)
Parafilm (Bemis, catalog number: PM999)
Equipment
Electrospinner (Glassman High Voltage, model: PS/FJS0R02.4)
Micro-miller (IKA Mini-Mill, model: 2836001)
Cold centrifuge (VWR, Eppendorf, model: 5430 R, catalog number: 76458-526)
Weigh scale (VWR, model: VWR-6001E, catalog number: 10204-996)
Analytical balance (VWR, model: VWR-310AC/CAL, catalog number: 89422-676)
Slide warmer with temperature control (4MD Medical, catalog number: CASCXH-2001)
Somnosuite low-flow anesthesia system (Kent Scientific, catalog number: 13-005-111)
Induction chamber (Kent Scientific, catalog number: Somno-0705)
Warming pad (Kent Scientific, catalog number: RT-0501)
Electric razor with vacuum (Remmington, catalog number: VPG-6530)
Tenotomy scissors (Fine Science Tools, catalog number: 14066–11)
Ridge-less forceps (Fine Science Tools, catalog number: 11016-17)
Germinator 500 (Braintree Scientific, catalog number: GER5287120V)
Procedure
文章信息
稿件历史记录
提交日期: Jul 22, 2024
接收日期: Sep 23, 2024
在线发布日期: Oct 17, 2024
出版日期: Nov 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Salazar-Puerta, A. I., Ott, N., Diaz-Starokozheva, L., Das, D., Lawrence, W. R., Johnson, J., Houser, R., Higuita-Castro, N., Stanford, K. I. and Gallego-Perez, D. (2024). A Protocol for co-Injecting Cells with Pulverized Fibers for Improved Cell Survival and Engraftment. Bio-protocol 14(22): e5117. DOI: 10.21769/BioProtoc.5117.
分类
生物工程 > 生物医学工程
医学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










