- EN - English
- CN - 中文
Preparing Chamber Slides With Pressed Collagen for Live Imaging Monolayers of Primary Human Intestinal Stem Cells
制备带压制胶原的腔室载玻片用于原代人肠道干细胞单层活细胞成像
发布: 2024年11月20日第14卷第22期 DOI: 10.21769/BioProtoc.5116 浏览次数: 2010
评审: Laxmi Narayan MishraSarah ShortAnu Thomas
Abstract
Primary human intestinal stem cells (ISCs) can be cultured and passaged indefinitely as two-dimensional monolayers grown on soft collagen. Culturing ISCs as monolayers enables easy access to the luminal side for chemical treatments and provides a simpler topology for high-resolution imaging compared to cells cultured as three-dimensional organoids. However, the soft collagen required to support primary ISC growth can pose a challenge for live imaging with an inverted microscope, as the collagen creates a steep meniscus when poured into wells. This may lead to uneven growth toward the center of the well, with cells at the edges often extending beyond the working distance of confocal microscopes. We have engineered a 3D-printed collagen mold that enables the preparation of chamber slides with flat, smooth, and reproducible thin collagen layers. These layers are adequate to support ISC growth while being thin enough to optimize live imaging with an inverted microscope. We present methods for constructing the collagen press, preparing chamber slides with pressed collagen, and plating primary human ISCs for growth and analysis.
Key features
• This protocol describes how to construct and use collagen presses for chamber slides, as demonstrated in Cotton et al. [1].
• The soft collagen and culture media presented are optimized for primary human intestinal stem cells.
• The full protocol, including 3D printing, preparing collagen-coated chamber slides, and plating cells can be completed in under one week.
• This protocol requires access to a 3D printer.
Keywords: Collagen press (胶原压制)Graphical overview
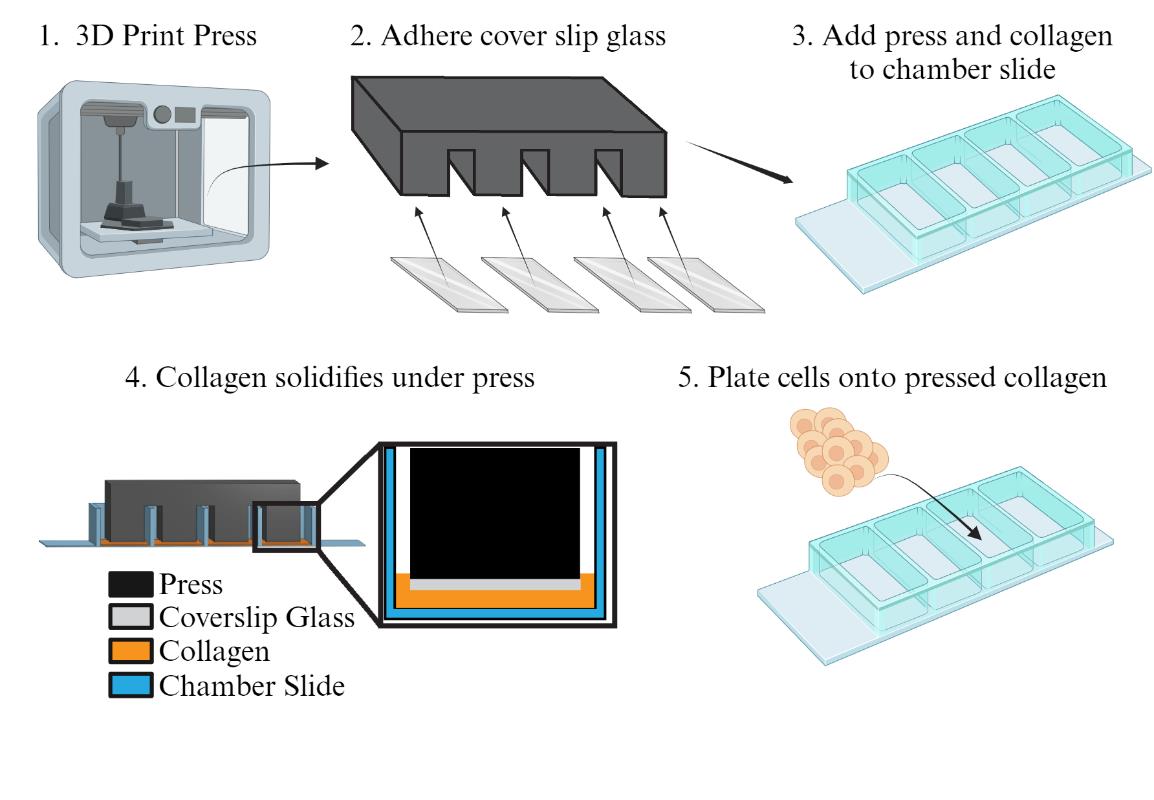
Preparing the collagen press and chamber slide for culturing human intestinal stem cells
Background
The intestinal epithelium is a dynamic tissue with differentiated cell types arising from a common crypt base columnar intestinal stem cell (ISC) [2]. Cells of the intestinal epithelium serve many roles, including absorbing nutrients, secreting mucus and hormones, providing a selectively permeable barrier between the body and luminal contents, and managing the microbiota. While much knowledge has been gleaned about the mammalian intestine using model organisms such as rodents, differences between the human intestine and those of model organisms are becoming increasingly known [3,4]. This makes it important to design systems for studying primary human intestinal cells. A common strategy for studying the human intestinal epithelium is to culture ISCs in a soft matrix and allow growth into 3-dimensional organoids (also referred to as enteroids) [5]. Organoids can be derived from primary crypts from donor epithelia [6] or via induced differentiation of pluripotent stem cells [7]. While organoids have greatly advanced our ability to study the human intestinal epithelium, they have limitations, such as an enclosed luminal compartment that is difficult to access and imaging challenges, as the cells grow in a spherical shape rather than in a flat plane. In 2017, a novel method for cultivating human small intestinal [8] or colon ISCs [9] as a self-replicating monolayer was introduced. We showed that primary cells grown on a soft collagen substrate (~9 kPa) in a growth medium rich in stem cell factors could be continuously propagated and passaged indefinitely [8]. Our recent work analyzes single-cell transcriptomic data to demonstrate that these growing monolayers largely consist of proliferating stem and progenitor cells, with minimal differentiated cells present [1]. Our optimized method for genetically modifying these primary human ISCs [10] to incorporate fluorescent reporter genes has enabled high-resolution analysis of various cell functions, including stemness [10], tight junctions [11], and the cell cycle [1]. However, we found that the thick collagen needed for maintaining primary ISC proliferation and stemness can also negatively affect high-resolution live imaging by adding distance between the growing cells and the microscope objective. When we used less collagen in a chamber slide to address this issue, we observed that the formation of a collagen meniscus at the edges of the chamber slides (Figure 1A) made consistent and clear imaging unfeasible. Cells in the thin central region of the well mostly exited the cell cycle and exhibited phenotypes indicative of auto-differentiation, while those in the thicker areas near the well walls proliferated but often risked growing beyond the working distance of our microscope (Figure 1B) [1]. To resolve this issue, we designed a collagen press that creates a flat, smooth collagen layer with a controlled and uniform thickness (Figure 1C). This innovation led to significantly more consistent cell growth and maintenance of stemness (Figure 1D). In this protocol, we describe how to design and construct collagen presses, how to pour reproducibly coated chamber slides, and how to culture and image primary human ISCs.
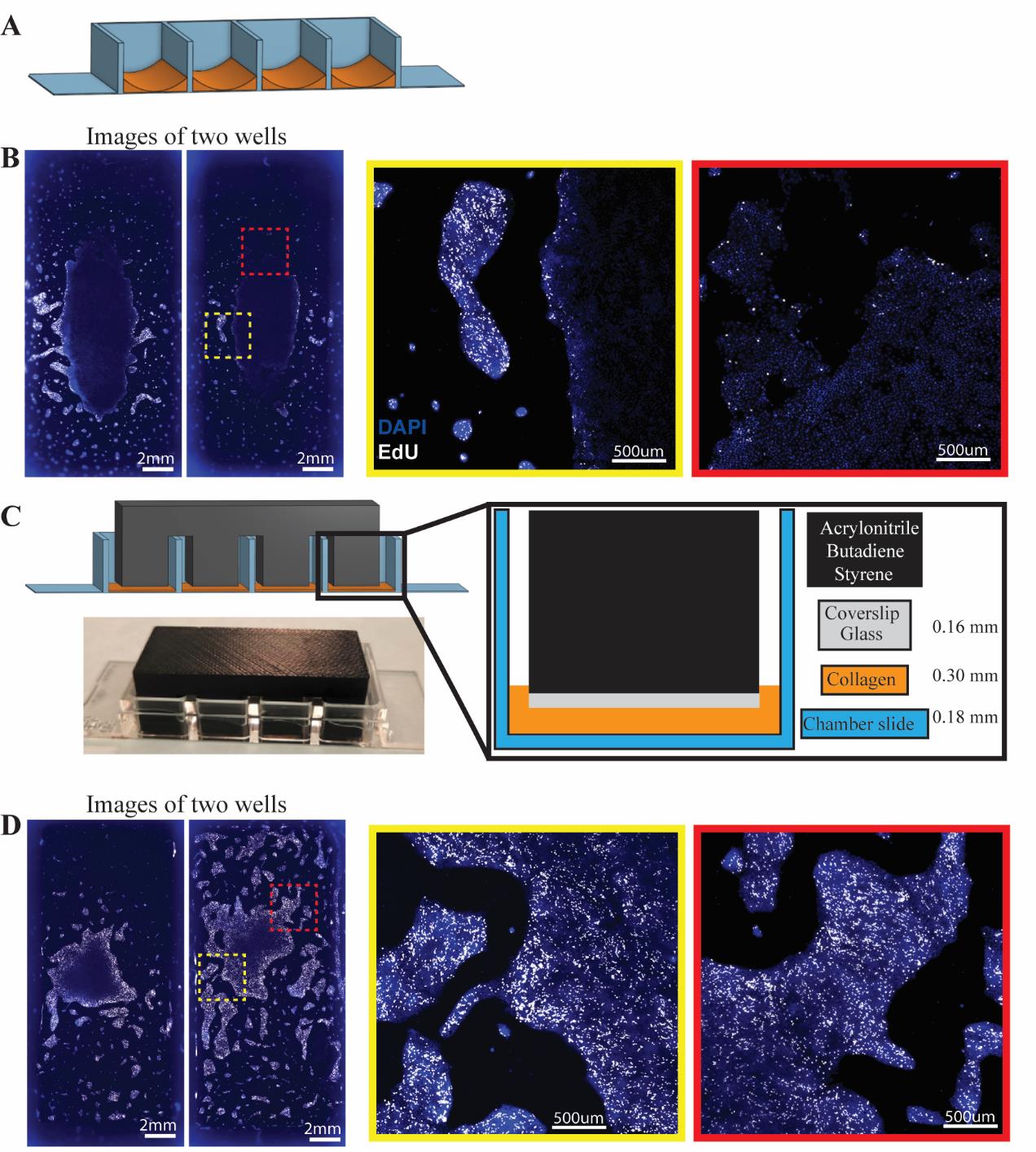
Figure 1. Effect of the collagen press on cell growth in chamber slide. A) Model of meniscus formation following freely poured collagen (orange) setting in chamber slides. B) Immunofluorescence of nuclei (DAPI, blue) and proliferation (EdU uptake, white) in two separate wells of intestinal stem cells (ISCs) in chamber slides poured with no press. Insets show differences in proliferative capacity and cellular spacing across areas of the same chamber well after three days of culture. C) Model for 3D-printed collagen press designed to sit across chamber slide walls and reach into wells to prevent meniscus formation. Coverslip glass glued to the leg bottoms forms a smooth surface. Photograph of a collagen press on a chamber slide. D) Immunofluorescence of nuclei (DAPI, blue) and proliferation (EdU uptake, white) in ISCs grown in two separate wells of a chamber slide using the 3D-printed collagen press. Insets indicate uniform proliferation across colonies. Adapted from Cotton et al. Scientific Reports [1]. © The Authors, some rights reserved; exclusive licensee, Springer Nature. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC) http://creativecommons.org/licenses/by-nc/4.0/.
Materials and reagents
Biological materials
LWRN Cells (ATCC, catalog number: CRL-3276)
Reagents
N-acetylcholine (NAC) (Sigma-Aldrich, catalog number: A9165)
Dulbecco's phosphate-buffered saline (dPBS) (Gibco, catalog number: 14190-144)
Na2HPO4 (Sigma, catalog number: S7907)
KH2PO4 (Sigma, catalog number: P5655)
NaCl (Sigma, catalog number: S5886)
KCl (Sigma, catalog number: P5405)
Sucrose (Fisher BP, catalog number: 220-1)
D-sorbitol (Fisher BP, catalog number: 439-500)
Y27632 (Selleck Chemical, catalog number: S6390)
DPBS (10×) (Gibco, catalog number: 14200-075)
Sodium bicarbonate (7.5%) (Gibco, catalog number: 25080-094)
Ethylenediaminetetraacetic acid (EDTA) (Corning, catalog number: 46-034-Cl)
Dithiothreitol (DTT) (Fisher Scientific BP, catalog number: 172-5)
Protease VIII (Sigma, catalog number: P5380)
Advanced DMEM/F12 (Gibco, catalog number: 12634-010)
Bovine serum albumin (Fisher Scientific, catalog number: BP1600-1)
Fetal bovine serum tetracycline negative (Gemini, catalog number: 100-800)
Primocin (InvivoGen, catalog number: ant-pm-05)
Gentamycin (Sigma-Aldrich, catalog number: G1914)
Amphotericin B (Sigma-Aldrich, catalog number: A2942)
Collagenase IV (Thermo Fisher, catalog number: LS004189)
TrypLE Express (Gibco, catalog number: 12605-010)
GlutaMAX (Thermo Fisher, catalog number: 35050061)
HEPES (Corning, catalog number: 25-060-CI)
Primocin (InvivoGen, VWR, catalog number: mspp-ant-pm2)
Pen/Strep (Thermo Fisher, catalog number: 15070063)
N-acetylcysteine (Sigma-Aldrich, catalog number: A9165)
EGF, murine (PeproTech, catalog number: 315-09)
Nicotinamide (Sigma-Aldrich, catalog number: N0636)
B27 supplement (Thermo Fisher, catalog number: 12587001)
Gastrin (Sigma-Aldrich, catalog number: G9145)
Prostaglandin E2 (PeproTech, catalog number: 3632464)
A 83-01 (Sigma-Aldrich, catalog number: SML0788)
SB202190 (PeproTech, catalog number: 1523072)
5-ethynyl-2'-deoxyuridine (EdU) (Molecular Probes, catalog number: C10634)
CuSO4 (Fisher Scientific, catalog number: S25286)
Sulfo-CY5-Azide (Lumiprobe, catalog number: A3330)
Ascorbic acid (Fisher Scientific, catalog number: AC352681000)
Cultrex rat collagen I (R&D Systems, catalog number: 3443-100-01)
Paraformaldehyde (Acros Organics, catalog number: 41678-0010)
DAPI (Invitrogen, catalog number: D3571)
NaOH (Thermo Fisher, catalog number: 12426)
NaHCO3 (Sigma-Aldrich, catalog number: S6014)
DPBS, 10× (Gibco, catalog number: 14200075)
Triton X-100 (MP Biomedicals, catalog number 02194854-CF)
Solutions
Collagen solution (see Recipes)
Neutralization buffer (see Recipes)
Small intestine (SI) maintenance media (see Recipes)
4% paraformaldehyde fixative (See Recipes)
EdU reaction buffer (see Recipes)
Sulfo-Cyanine5-azide (see Recipes)
Permeabilization solution (see Recipes)
EdU staining solution (see Recipes)
DAPI solution (see Recipes)
Recipes
Collagen solution
Note: Neutralization buffer can be made in advance and stored at 4 °C for up to one month.
Reagent Final concentration Volume for 12 mL total Rat collagen I (3 mg/mL) 1 mg/mL 4 mL Neutralization buffer (Recipe 2) - 8 mL Neutralization buffer
Note: NaOH is added to neutralize the acetic acid in the Collagen I solution. The values here are for using Cultrex rat collagen supplied at 3 mg/mL in 20 mM acetic acid. The amount of NaOH will vary if collagen stocks with other concentrations are used. NaOH should be used at a 1.15 molar equivalent to the acetic acid added with the collagen.
Reagent Final concentration Volume for 8 mL total 10× dPBS 1× 1.2 mL HEPES (1 M) 0.02 M 0.24 mL NaHCO3 (7.5%) 0.45% w/v 0.72 mL NaOH (1 N) see note 0.092 mL DI water 50% total volume 6 mL Small intestine (SI) maintenance media
Note: All reagents, except the conditioned media and mEGF, can be mixed together separately to make a “Basal Media” that can be kept at 4 °C for up to one month. Then, to make SI maintenance media, use basal media and conditioned media at a 1:1 ratio, with mEGF added at 1:20,000.
*Note: The protocol for preparing L-WRN conditioned media has been described previously [12,13]
Reagent Final concentration Volume for 50 mL total L-WRN conditioned media* 50% 25 mL Adv DMEM/F12 - 22.5 mL B-27 (without Vit A) 1× 1 mL Nicotinamide (1 M) 10 mM 0.5 mL HEPES (1 M) 10 mM 0.5 mL GlutaMAX (100×) 0.5× 0.25 mL Pen/Strep (100×) 0.5× 0.25 mL N-acetyl cysteine (NAC, 0.5 M) 1.25 mM 0.125 mL Primocin (50 mg/mL) 50 µg/mL 0.05 mL SB202190 (30 mM) 3 µM 5 µL Gastrin I, human (100 μM) 10 nM 5 µL Prostaglandin E2 (PGE2, 1 mM) 10 nM 0.05 µL Murine EGF (1 mg/mL) 50 ng/mL 2.5 µL 4% paraformaldehyde fixative
Note: Add paraformaldehyde powder to 800 mL of PBS, start a stir bar spinning, then warm to just below 70 °C. Once all solids have dissolved, turn off the heat, allow to cool, filter the solution, and then bring to 1 L with PBS.
The final product can be stored at -20 °C indefinitely; the thawed solution should be used within two weeks.
Reagent Final concentration Volume for 1 L total Paraformaldehyde 4% 40 g PBS - To 1 L EdU reaction buffer
Note: Can be stored at 4 °C indefinitely.
Reagent Final concentration Volume for 38 mL total 10× dPBS 2.6× 10 mL DI water - 28 mL Sulfo-Cyanine5-azide (0.8 mM)
Note: Can be stored at -20°C indefinitely.
Reagent Final concentration Volume Sulfo-Cyanine5-azide 0.8 mM 1 mg DI water - 1.318 mL Permeabilization solution
Note: Can be stored at 4°C indefinitely.
Reagent Final concentration Volume Triton-X100 0.5% 1 mL DI water - 200 mL EdU staining solution
Reagent Final concentration Volume for 1 mL total EdU reaction buffer (2.63× PBS) 2× 760 µL CuSO4 (100 mM) 0.02 M 40 µL Sulfo-Cyanine5-azide (0.8 mM) 0.45% w/v 2.5 µL L-ascorbic acid (1 M) 0.2 M 200 µL DAPI solution
Note: 5 mg/mL DAPI can be stored at -20 °C indefinitely. Avoid repeated freeze/thaws. Make the final product fresh on the day of use.
Reagent Final concentration Volume for 38 mL total DAPI (5 mg/mL in DI water) 5 µM 1 µL PBS - 10 mL
Laboratory supplies
Chlorinated polyethylene
Cover glass thickness 1, 18 × 18 mm (Corning, catalog number: 2845-18)
Glass glue (Loctite)
15 mL conical tube (Corning, catalog number: CLS430790)
Costar Stripette, 5 mL (Corning, catalog number: 4487)
µ-Slide 4 Well ibiTreat chamber slides (Ibidi, catalog number: 80426)
Rain-X glass water repellent (Rain-X, catalog number: 800002250)
Equipment
Ultimaker S3 3D printer (Ultimaker)
Engraving pen (Millipore Sigma, catalog number: Z225568)
Incubator set to 37 °C and 5% CO2
Water bath set to 37 °C
Clinical centrifuge capable of spinning 15 mL conical tubes at least 800× g
Confocal microscope with environmental chamber
For fluorescence live imaging of PIP-H2A cell cycle reporter lines [1], we used an Andor Dragonfly spinning disk confocal microscope mounted on a Leica DMi8 microscope stand, using a Leica HC PL APO 20×/0.75 LWD air objective with pinhole size set to 40 μm. Light was collected with a 593/43 Semrock emission filter using a HC Fluotar L 25×/0.95 W 0.17 VISIR objective or a 514 nm laser was used for excitation using a 538/20 Semrock emission filter.
Procedure
文章信息
稿件历史记录
提交日期: Aug 14, 2024
接收日期: Sep 27, 2024
在线发布日期: Oct 15, 2024
出版日期: Nov 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Burclaff, J. and Magness, S. T. (2024). Preparing Chamber Slides With Pressed Collagen for Live Imaging Monolayers of Primary Human Intestinal Stem Cells. Bio-protocol 14(22): e5116. DOI: 10.21769/BioProtoc.5116.
- Cotton, M. J., Ariel, P., Chen, K., Walcott, V. A., Dixit, M., Breau, K. A., Hinesley, C. M., Kedziora, K. M., Tang, C. Y. and Zheng, A. (2024). An in vitro platform for quantifying cell cycle phase lengths in primary human intestinal epithelial cells. Sci Rep. 14(1): 15195.
分类
干细胞 > 成体干细胞 > 肠道干细胞
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link