- EN - English
- CN - 中文
Small-Molecule Probe for Imaging Oxidative Stress–Induced Carbonylation in Live Cells
小分子探针用于活细胞氧化应激诱导羰基化成像
发布: 2024年11月20日第14卷第22期 DOI: 10.21769/BioProtoc.5112 浏览次数: 1575
评审: Komuraiah MyakalaThomas LintoAnonymous reviewer(s)
Abstract
Protein carbonylation has been known as the major form of irreversible protein modifications and is also widely used as an indicator of oxidative stress in the biological environment. In the presence of oxidative stress, biological systems tend to produce large amounts of carbonyl moieties; these carbonyl groups do not have particular UV-Vis and fluorescence spectroscopic characteristics that we can differentiate, observe, and detect. Thus, their detection and quantification can only be performed using specific chemical probes. Commercially available fluorescent probes to detect specific carbonylation in biological systems have been used, but their chemical portfolio is still very limited. This protocol outlines the methods and procedures employed to synthesize a probe, (E,Z)-2-(2-(2-hydroxybenzylidene)hydrazonyl)-5-nitrophenol (2Hzin5NP), and assess its impact on carbonylation in human cells. The synthesis involves several steps, including the preparation of its hydrazone compounds mimicking cell carbonyls, 2-Hydrazinyl 5-nitrophenol, (E,Z)-2-(2-ethylidenehydrazonyl)-5-nitrophenol, and the final product (E,Z)-2-(2-(2-hydroxybenzylidene)hydrazonyl)-5-nitrophenol. The evaluation of fluorescence quantum yield and subsequent cell culture experiments are detailed for the investigation of 2Hzin5NP effects on cell proliferation and carbonylation.
Key features
• This protocol builds upon probe development using click chemistry method by Dilek et al. [1], and its biolabeling application in renal cancer cell lines.
• The non-fluorescent probe has a fast reaction with carbonyl moieties at neutral pH to form a stable fluorescent product leading to a spectroscopic alteration.
• Microscopic and fluorometric analyses can distinguish the exogenous and endogenous ROS-induced carbonylation profile in human dermal fibroblasts along with renal cell carcinoma.
• Carbonylation level that differs in response to exogenous and endogenous stress in healthy and cancer cells can be detected by the newly synthesized fluorescent probe.
Keywords: Bioorthogonal chemistry (生物正交化学)Graphical overview
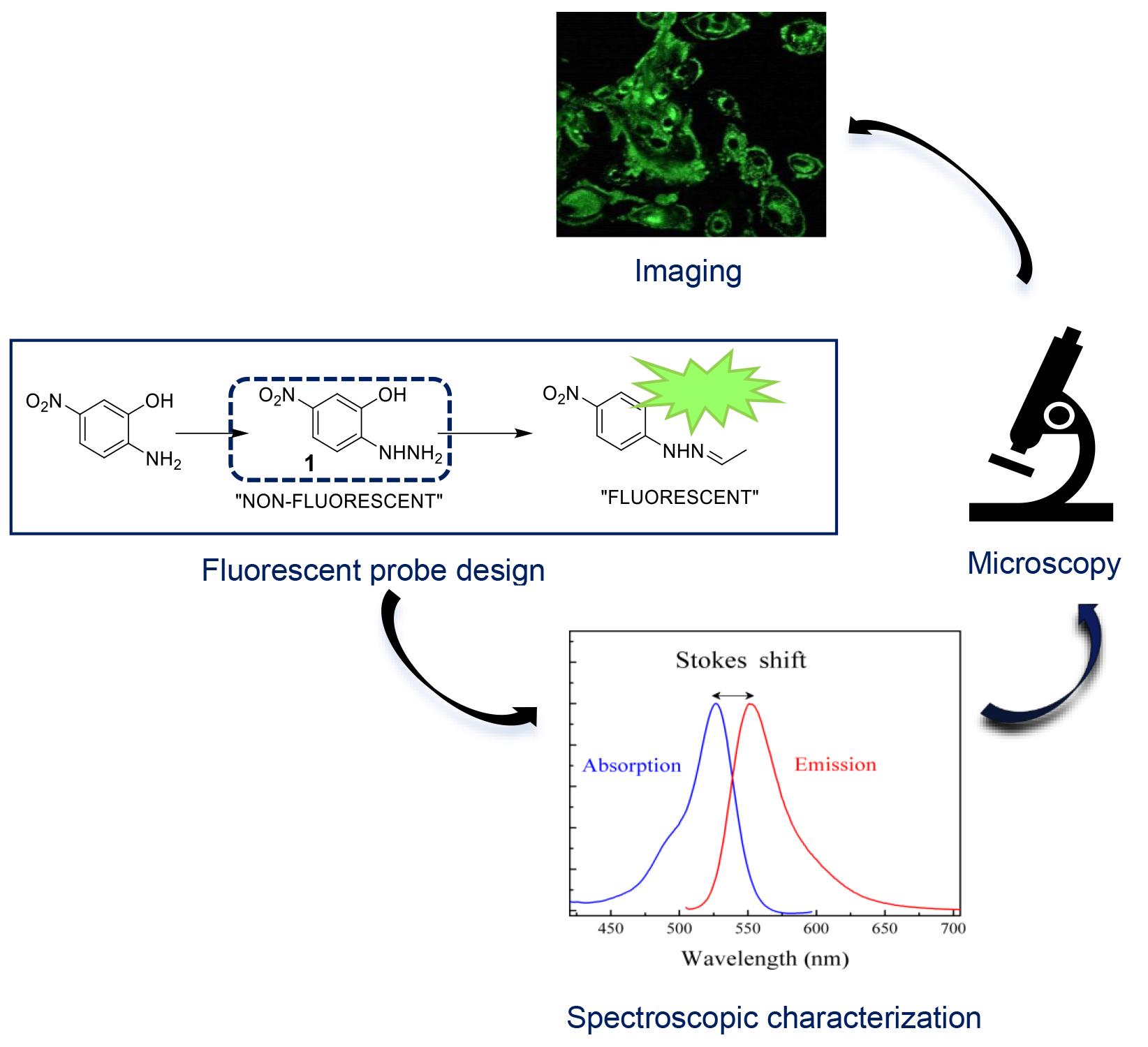
Background
Fluorescent probes play a crucial role in visualizing cellular events; the development of probes for imaging oxidative stress-induced carbonylation in live cells represents a significant area of research [1–4]. Oxidative stress–induced protein carbonylation is a key marker of cellular damage and is implicated in various pathological conditions, including neurodegenerative diseases and cancer [5–7]. This area of study aims to enhance our understanding of cellular responses to oxidative stress [8] and provides potential targets for therapeutic interventions [9,10]. Several methodologies have been employed to study protein carbonylation, including immunoblotting and mass spectrometry [11]. However, these techniques often lack spatiotemporal resolution and may require cell fixation, preventing real-time observation. Fluorescent probes, therefore, offer the advantage of live-cell imaging, allowing dynamic monitoring of carbonylation processes [12,13]. To monitor carbonylation in live-cell imaging, the click chemistry method [14,15] is often used as a fast conjugation method, which can be characterized by high efficiency, selectivity, and compatibility with biological systems [16], exemplified by the hydrazine-hydrazone chemistry, where the reactive hydrazine group reacts with an aldehyde or ketone moiety to form a stable hydrazone linkage [17]. This chemistry has been widely employed in the development of fluorescent probes, such as utilizing a fluorescent dye conjugated with an aldehyde group that selectively reacts with a hydrazine-functionalized biomolecule, enabling precise visualization and tracking within biological systems. Our protocol employs a fluorescent probe, (E,Z)-2-(2-(2-hydroxybenzylidene)hydrazonyl)-5-nitrophenol (2Hzin5NP), facilitating real-time observation of oxidative stress–induced carbonylation in live cells. The protocol includes a WST-1 cell proliferation assay and quantification of carbonylation in cell lysate, enabling quantitative assessment of cellular responses. One particular limitation of using this probe for carbonylation imaging is its specificity for responding to an oxidative stress environment; this should be validated against other cellular processes. The protocol involves detecting carbonylation levels in renal carcinoma cell lines A-498 and ACHN, as well as in human dermal fibroblasts (HDF), using these as cell models [18]. However, its applicability to other cell types should also be investigated. Beyond imaging oxidative stress–induced carbonylation, this protocol may find applications in drug discovery and cellular signaling such as assessing the impact of potential therapeutics on oxidative stress–induced cellular damage and investigating the role of carbonylation in cellular signaling pathways. In conclusion, the presented protocol offers a valuable tool for researchers in the field of oxidative stress, enabling live-cell imaging of carbonylation with potential applications in various scientific domains. The stepwise synthesis and quantitative analysis contribute to the protocol's robustness, though ongoing research will refine its specificity and broaden its applicability.
Materials and reagents
Cell lines
A-498, primary human kidney epithelial carcinoma, adherent (ATCC, catalog number: Htb-44)
ACHN, metastatic renal cell adenocarcinoma, adherent (ATCC, catalog number: Crl-1611)
HDF, human dermal fibroblast, adherent (ATCC, catalog number: PCS 201-012)
Chemicals
Chemical synthesis
Silica gel (silica gel 60-200 mesh) (2.5 kg) (Merck, catalog number: 107734)
Hexane (2.5 L) (Merck, catalog number: 104368)
Ethanol (EtOH) (100%) (Merck, catalog number: M.100986.2500)
Chloroform (Merck, catalog number: SC.CL.0200.2500)
Dichloromethane (2.5 L) (Merck, catalog number: M.106050.2500)
Acetone (2.5 L) (Merck, catalog number: M.100013.2500)
Hydrochloric acid (HCl) (2.5 L) (Merck, catalog number: 100317)
Sodium hydroxide pellets (Merck, catalog number: 106498)
Sodium chloride (VWR, catalog number: SC.SO.0227.1000)
Methanol (spectral grade, anhydrous) (2.5 L) (Merck, catalog number: 106009)
Dioxane (1 L) (Spectrophotometric, catalog number: 154822)
Methanol (normal, 2.5 L) (Sigma-Aldrich, catalog number: 34885)
Sodium nitrite (1 kg) (Merck, catalog number: 106544)
Tin (II) chloride (Stannous chloride) (100 g) (Fluka, catalog number: 31669)
2-Amino-5-Nitrophenol (100 g) (Sigma, catalog number: 303585)
Diethyl ether (1 L) (Merck, catalog number: 100921)
TLC aluminum sheets (Merck, catalog number: 105554)
Cell culture media
Dulbecco’s modified Eagle’s medium (DMEM), high glucose (Gibco, catalog number: 41966)
Fetal bovine serum (FBS), cell culture tested (Gibco, catalog number: 10082)
Other reagents for cell culture
Bovine serum albumin (BSA), protein standard (Sigma, catalog number: P0834)
Dimethyl sulfoxide (DMSO) (Santa Cruz, catalog number: Sc-202581)
Dulbecco’s phosphate buffered saline (PBS) (Pan Biotech, catalog number: P04-53500)
H2O2 (50 wt % in H2O) (Sigma, catalog number: 519813)
L-Glutamine (Invitrogen, catalog number: 25030)
Methanol 99% (Sigma, catalog number: 34885)
Phenylmethanesulfonylfluoride (PMSF) (Sigma, catalog number: 78830)
Protease inhibitor (Pi) (Sigma, catalog number: P8340)
Penicillin-streptomycin (Thermo Scientific, catalog number: Sv30010 or Biochrom, catalog number: A2213)
Trypsin-EDTA (Biochrom, catalog number: L2153)
Kits
Cell Proliferation Reagent WST-1 (Roche, catalog number: 05015944001)
Protein Assay Reagent A (Bio-Rad, catalog number: 5000113)
Protein Assay Reagent B (Bio-Rad, catalog number: 5000114)
DCFDA, Cellular Reactive Oxygen Species Detection Assay Kit (Abcam, catalog number: Ab113851)
Small equipment and other supplies
Bright-LineTM hemocytometer (Sigma-Aldrich, catalog number: Z359629)
Coverslip (Sigma-Aldrich, catalog number: Z375357)
Electronic pipette (CAPP Aid)
Filter 0.22 mm (TPP), 0.45 mm (Santorium Stedim Biotech)
Graduated cylinder 50, 250, 500, 1,000 mL (Isolab)
Micropipettes 10, 20, 100, 200, 1,000 μL (Eppendorf Research)
Pipette tips 10, 100, 200, 1,000 µL (Capp Expell Plus)
Polypropylene centrifuge tubes 0.5, 1.5, 2, 15, 50 mL (Isolab)
Serological pipettes 2, 5, 10, 25 mL (Grenier Bio or Axygen)
Tissue culture flasks, T-25, T-75, T-150 (TPP or Grenier-Bio)
Multiple-well cell culture plates and cryovials (TPP or Grenier-Bio)
Whatman paper (Isolab)
Equipment
-80 °C freezer (Thermo, model: Forma -86 C ULT Freezer)
Bruker Avance III 500 MHz Spectrometry
Centrifuge (Hettich, model: Mikro 22r and Sigma, model: 2-5 Centrifuge)
CO2 Incubator (Nuaire, model: Nu5510/E/G)
Confocal microscope (Zeiss, model: Lsm 800)
Fluorescence microscope (Nikon, model: 80i Eclipse Fluorescence Microscope)
Fume Hood (Greenlab)
Heater (Bioer, model: Mb102)
Laminar flow cabinet (ESCO Lab culture Class II Biohazard Safety Cabinet 2A)
Light microscope (Nikon, model: Eclipse #Ts100)
Magnetic stirrer (Heidolph, model: Mr 3004)
pH meter (Hanna Instruments, model: Ph211)
Rotary evaporator (Heidolph, model: Hei-VAP Silver Packages)
UV lamb, cabinet (CAMAG)
Varioskan Lux multimode microplate reader (Thermo Fisher)
Vortex (Stuart Sa8p)
Water bath (Stuart, model: Sb540)
Software and datasets
GraphPad Prism 6
Free versions of SigmaPlot Software and ChemDraw
Procedure
文章信息
稿件历史记录
提交日期: Apr 5, 2024
接收日期: Sep 25, 2024
在线发布日期: Oct 15, 2024
出版日期: Nov 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Dilek, O., Telci, D. and Erkan-Candag, H. (2024). Small-Molecule Probe for Imaging Oxidative Stress–Induced Carbonylation in Live Cells. Bio-protocol 14(22): e5112. DOI: 10.21769/BioProtoc.5112.
- Erkan, H., Telci, D. and Dilek, O. (2020). Design of Fluorescent Probes for Bioorthogonal Labeling of Carbonylation in Live Cells. Sci Rep. 10(1): 7668.
分类
生物物理学 > 显微技术 > 双光子激光扫描显微镜
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link