- EN - English
- CN - 中文
Preparation of Polarity-Marked Microtubules Using a Plus-End Capping DARPin
利用正端封闭DARPin制备极性标记微管
发布: 2024年11月20日第14卷第22期 DOI: 10.21769/BioProtoc.5109 浏览次数: 1651
评审: Manish ChamoliMarcus Braun
Abstract
The eukaryotic cytoskeleton is formed in part by microtubules, which are relatively rigid filaments with inherent structural polarity. One consequence of this polarity is that the two ends of a microtubule have different properties with important consequences for their cellular roles. These differences are often challenging to probe within the crowded environment of the cell. Fluorescence microscopy–based in vitro assays with purified proteins and stabilized microtubules have been used to characterize polarity-dependent and end-specific behaviors. These assays require ways to visualize the polarity of the microtubules, which has previously been achieved either by the addition of fluorescently tagged motor proteins with known directionality or by fluorescently polarity marking the microtubules themselves. However, classical polarity-marking protocols require a particular chemically modified tubulin and generate microtubules with chemically different plus and minus segments. These chemical differences in the segments may affect the behavior of interacting proteins of interest in an undesirable manner. We present here a new protocol that uses a previously characterized, reversibly binding microtubule plus-end capping protein, a designed ankyrin repeat protein (DARPin), to efficiently produce polarity-marked microtubules with different fluorescently labeled, but otherwise biochemically identical, plus- and minus-end segments.
Key features
• Produces polarity-marked microtubules with biochemically identical segments.
• Allows analysis of end-specific and polarity-dependent activities of purified microtubule-associated proteins
• Requires purified microtubule plus-end capping DARPin (D1)2
• Concentrations optimized for porcine brain tubulin
Keywords: Microtubules (微管)Graphical overview
Background
Microtubule filaments have a structural polarity determined by the orientation of their constituent α/β-heterodimers. When grown in vitro from purified tubulin, microtubules stochastically switch between growing and shrinking at both ends with distinct dynamics [1,2]. Plus-ends, where β-tubulin is exposed, typically grow faster and switch to the shrinking state more frequently than the minus-ends [2,3]. In cells, where a growing list of proteins has been shown to preferentially interact with plus- or minus-microtubule ends [4–6], the minus-end is often, but not always, stabilized by the microtubule nucleating γ-tubulin ring complex [7–9], whereas the plus-end is subject to control by various proteins that control dynamic instability rates [4]. However, the extent to which microtubule-associated proteins selectively interact with one or the other microtubule end is often hard to determine. This is due to the high density of microtubules in structures such as the mitotic spindle or in neuronal axons relative to the resolution of fluorescence microscopy typically used to observe proteins in live cells.
Biochemical assessment in vitro, with purified proteins of interest, can readily reveal end-specific behaviors, amenable to straightforward kinetic and dynamic analyses that are often impossible in cells [4,10–17]. Determining this behavior requires being able to determine unambiguously the polarity of individual microtubules, which is often done through differential fluorescent marking [18–20]. However, the majority of protocols published for selective marking involve use of tubulin chemically modified with N-ethylmaleimide (NEM) [18,19,21], which blocks microtubule growth from the minus-end but is also inhibitory to growth at the plus-end, even if less so, requiring careful optimization of modified and unmodified tubulin concentrations and resulting in microtubules that are not fully polymerization-competent in subsequent assays. Furthermore, the protocols involve two steps with two different stabilizing agents, with highly distinct mechanisms of stabilization—the slowly-hydrolyzing GTP analog guanosine-5'-[(α,β)-methyleno]triphosphate (GMPCPP) and the microtubule-binding drug paclitaxel (taxol). This means that the two segments of the microtubule lattice, as well as the ends, are also distinct—one segment contains only GMPCPP-tubulin, while the other contains only taxol-stabilized, partially NEM-modified GDP-tubulin, with differences in chemistry, structure, and mechanical properties [22–27]. These differences can confound the analysis of protein binding to the different microtubule segments and the behavior of potentially end-specific microtubule-binding proteins. Indeed, various microtubule-associated proteins are known to exhibit strikingly different behaviors depending on the nucleotide state of the microtubule lattice [28–31].
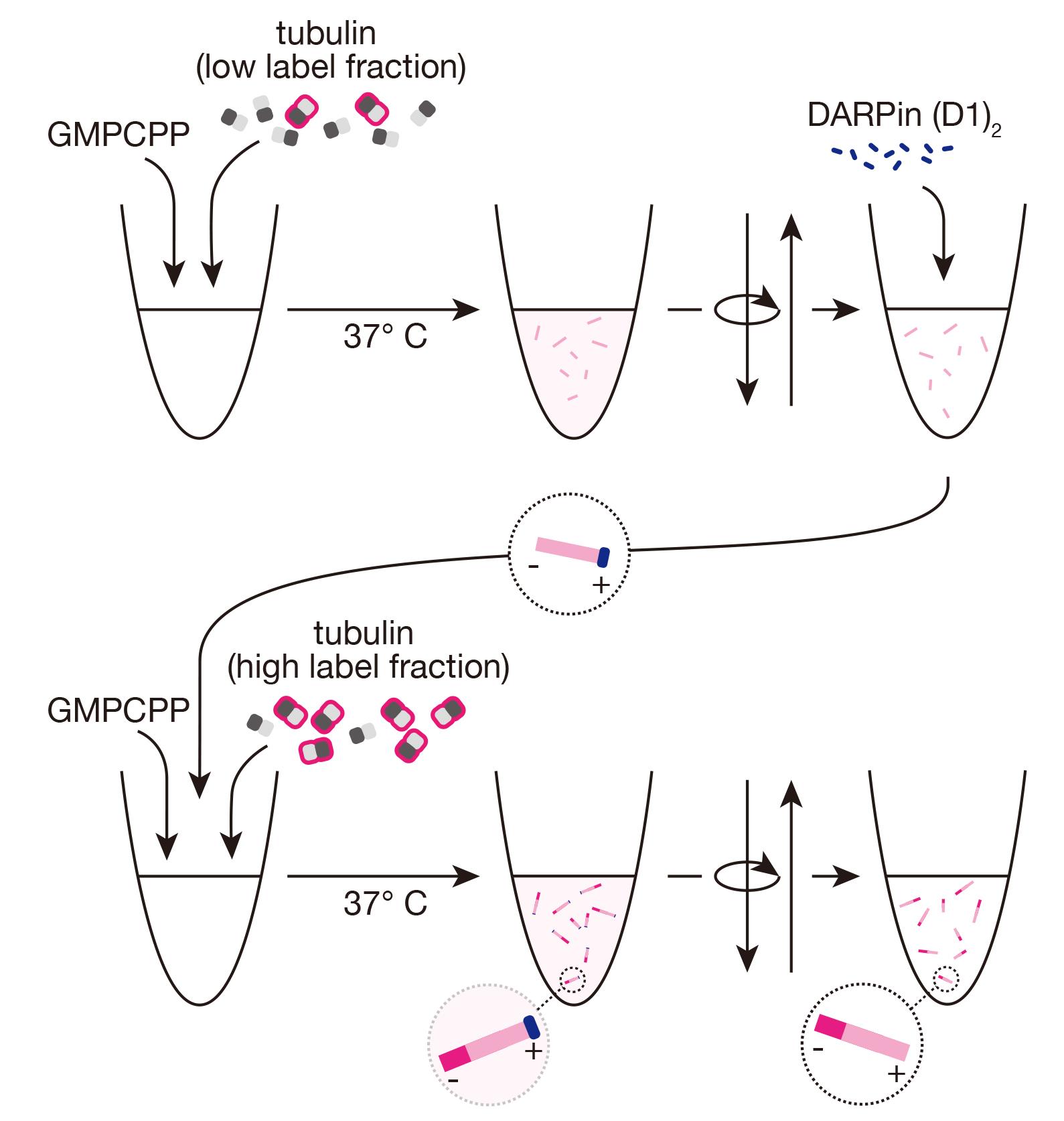
We were interested in updating this protocol in such a way that we could produce polarity-marked microtubules where both segments were otherwise biochemically identical. Our procedure is based on recent work that has identified de novo–designed proteins that reversibly cap the growth of distinct microtubule ends [32–34]. We use one of these proteins, a designed ankyrin repeat protein (DARPin) in a tandem arrangement, termed (D1)2, to provide a new method for polarity marking stable microtubules that are grown with GMPCPP-only throughout. Furthermore, we show how to confirm the polarity of the microtubules, using a simple gliding assay with a microtubule motor protein of known directionality.
Materials and reagents
Reagents
Porcine brain tubulin, prepared as previously reported [35,36]
Purified tandem DARPin D1 protein [(D1)2], expressed in E. coli and purified according to standard DARPin expression and purification protocols [37,38]. A detailed description of the (D1)2 expression on a 500 mL scale and purification via metal affinity chromatography and size exclusion chromatography can be found here [14,32]. The bacterial (D1)2 expression plasmid can be requested from A. Pluckthun (plueckthun@bioc.uzh.ch)
Fluorescently labeled porcine brain tubulin (i.e., AlexaFluor647 or Atto647-labeled) prepared as previously reported [21,35]
Note: Fluorescence labeling ratio should be determined by Nanodrop prior to starting the protocol.
Optional for neutravidin-biotin-based immobilization in, e.g., TIRF microscopy assays: biotinylated porcine brain tubulin, prepared as previously reported [21,35]
Piperazine-N,N'-bis(2-ethanesulfonic acid) (PIPES) (Sigma-Aldrich, catalog number: P6757)
Magnesium chloride (MgCl2), 1 M (Sigma-Aldrich, catalog number: 63069)
Ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA) (Sigma-Aldrich, catalog number: E3889)
Guanosine-5'-[(α,β)-methyleno]triphosphate (GMPCPP), 10 mM (Jena bioscience, catalog number: NU-405)
High-quality purified water, e.g., Millipore Milli-Q
For gliding assay:
An active, directional microtubule motor protein; we had the minus-end-directed HSET motor protein available in the laboratory, prepared as previously published [39]. Many other microtubule motors with established polarity, such as the plus-end directed kinesin-1 or its motor-domain truncations, can be used instead
Poly(L-lysine)-polyethylene glycol (PLL-PEG) [SuSoS, catalog number: PLL(20)-g[3.5]- PEG(2)]
β-Mercaptoethanol (β-ME), 14.3 M (Sigma-Aldrich, catalog number: M6250)
β-casein (Sigma-Aldrich, catalog number: C6905)
ATP (Sigma-Aldrich, catalog number: A6419)
EGTA, 0.5 M, pH 8 (see Recipes)
BRB80, 5× stock (see Recipes)
BRB80, 1× (see Recipes)
For gliding assay:
PLL-PEG, 2 mg/mL (see Recipes)
β-casein, 25 mg/mL (see Recipes)
ATP, 100 mM, pH 7 (see Recipes)
Gliding buffer (see Recipes)
Recipes
Note: All solutions should be prepared using high-quality purified water, e.g., Millipore Milli-Q.
EGTA, 0.5 M, pH 8
Add solid KOH to dissolve EGTA powder and adjust pH only by adding KOH, being careful not to overshoot; store at room temperature.
BRB80, 5× (50 mL)
Reagent Final concentration Quantity or Volume PIPES 400 mM 6.06 g EGTA (0.5 M, pH 8.0) 5 mM 0.5 mL MgCl2 (1 M) 5 mM 0.25 mL Total 50 mL, pH 6.8 Filter (0.22 μm pore size) and store for up to 4 weeks at 4 °C.
Note: PIPES dissolves upon the addition of solid KOH. MgCl2 and EGTA should be added only after PIPES is dissolved (requiring ~1.35 g of KOH for these amounts). The final pH should be adjusted only by addition of KOH, being careful not to overshoot, to control the total ionic strength of the solution.
BRB80, 1×
Diluted from the above recipe on the day of use; keep on ice.
PLL-PEG, 2 mg/mL, in water
Aliquot and store at -20 °C.
β-casein, 25 mg/mL, in BRB80
Ultracentrifuge, aliquot, snap-freeze in liquid nitrogen, and store at -80 °C.
ATP, 100 mM, pH 7
Filter, aliquot, and store at -80 °C.
Gliding buffer
BRB80 with 5 mM β-ME, 1 mM ATP, 1 mg/mL β-casein; prepare on the day of the experiments and keep at room temperature.
Laboratory supplies
1.5 mL centrifuge tubes (e.g., Eppendorf, catalog number: 0030120086)
Ice bucket
Pipettes
Pipette tips
For imaging/ gliding assay:
Cover glasses, thickness #1.5, 22 × 22 mm (VWR, catalog number: 630-1843)
Standard microscopy slides, 76 × 26 mm (e.g., VWR, catalog number: 631-1550P)
Double-sided adhesive fleece transparent tape, 10 m × 15 mm (Tesa®, catalog number: 053380000001)
Filter paper 3 mm CHR, 46 cm × 57 cm (Whatman®, catalog number: 3030-917)
KorasilonTM Paste, medium viscosity (Sigma-Aldrich, catalog number: 769681-1EA)
Diamond scribing pen (e.g., Ted Pella, catalog number: 54468)
Forceps (e.g., Sigma-Aldrich, catalog number: Z680214-1EA)
Equipment
Microcentrifuge (e.g., Eppendorf, model: 5418 R)
Heat block set to 37 °C (e.g., Eppendorf, model: ThermoStat C)
For imaging/ gliding assay:
Fluorescence microscope (e.g., Nikon Instruments, model: E600)
Software and datasets
ImageJ/FIJI (e.g., ImageJ version 1.54, https://imagej.net/ij/index.html)
Procedure
文章信息
稿件历史记录
提交日期: Jul 2, 2024
接收日期: Sep 13, 2024
在线发布日期: Oct 17, 2024
出版日期: Nov 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Henkin, G., Brito, C., Plückthun, A. and Surrey, T. (2024). Preparation of Polarity-Marked Microtubules Using a Plus-End Capping DARPin. Bio-protocol 14(22): e5109. DOI: 10.21769/BioProtoc.5109.
- Henkin, G., Brito, C., Thomas, C. and Surrey, T. (2023). The minus-end depolymerase KIF2A drives flux-like treadmilling of γTuRC-uncapped microtubules. J Cell Biol. 222(10). https://doi.org/10.1083/jcb.202304020.
分类
细胞生物学 > 细胞结构 > 微管
生物化学 > 蛋白质 > 标记
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link