- EN - English
- CN - 中文
The on-Site Monitoring and Specimen-Making of Ectoparasites on Rodents and Other Small Mammals
啮齿动物及其他小型哺乳动物体外寄生虫的现场监测与标本制作
发布: 2024年11月05日第14卷第21期 DOI: 10.21769/BioProtoc.5104 浏览次数: 1562
评审: Zeeshan BandaySatya Ranjan SahuAnonymous reviewer(s)
Abstract
The ectoparasites of rodents and other small mammals usually involve five categories of arthropods—fleas, sucking lice, gamasid mites, chigger mites, and occasionally, ticks. These ectoparasites are medically important, serving as vectors for diseases such as plague, murine typhus, scrub typhus, forest encephalitis, Lyme disease, and other zoonoses. Field surveys, collection, and specimen preparation of ectoparasites are crucial for studying taxonomy, faunistics, ecology, and epidemiology. They are also essential for vector surveillance. The present protocol summarizes the on-site monitoring and specimen-making of ectoparasites of rodents and other sympatric small mammals. Besides the collection and specimen preparation of small mammal hosts, the protocol describes in detail the collection, fixation, specimen-making, and taxonomic identification of ectoparasites and provides some monitoring indices. The on-site monitoring indices include the host density index and the infestation indices of ectoparasites (prevalence, mean abundance, mean intensity). The methodologies outlined in this protocol provide technical guidance and references for vector monitoring (surveillance) and control.
Key features
• Collection and specimen preparation of small mammal hosts, including rodents (rats, mice, and voles) and other sympatric small mammals (shrews, tree shrews, and pikas)
• Collection, fixation, specimen-making, and taxonomic identification of ectoparasites, including fleas, sucking lice, gamasid mites, chigger mites, and ticks
• On-site monitoring indices—host density index and infestation indices of ectoparasites: prevalence, mean abundance, and mean intensity
Keywords: Rodent (啮齿动物)Graphical overview
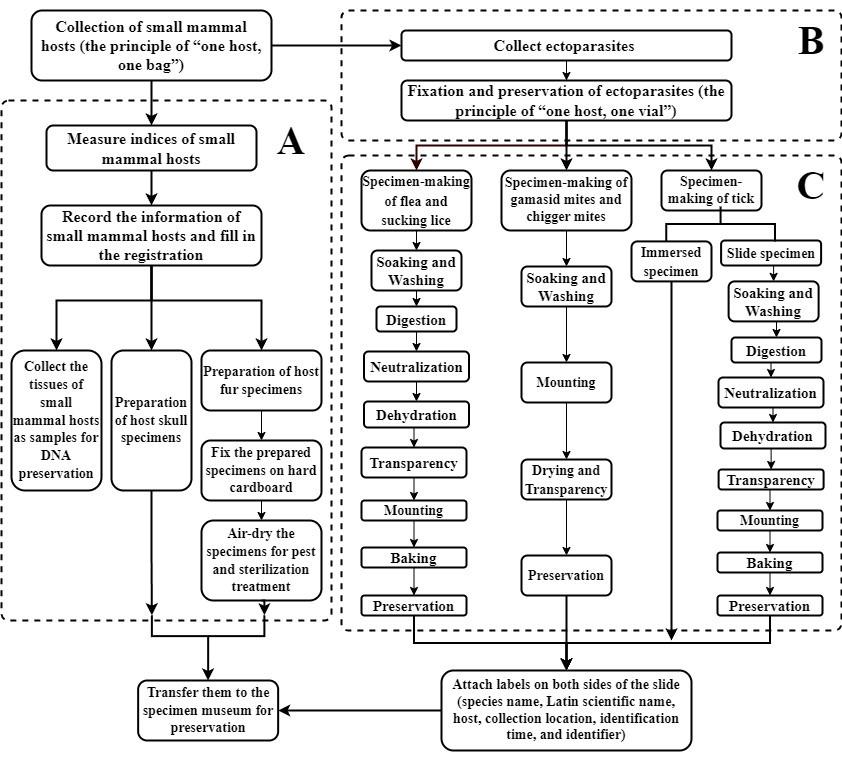
Flowchart for collection and specimen-making of ectoparasites and their hosts. A. Process for the collection and specimen preparation of small mammal hosts. B. Collection and fixation of ectoparasites. C. Process for specimen-making for different categories of ectoparasites.
Background
Rodents (rats, mice, and voles) and other sympatric small mammals (shrews, tree shrews, and pikas) often share habitats. They serve as significant infectious sources and reservoir hosts for numerous zoonotic diseases, including plague, murine typhus, scrub typhus, and hemorrhagic fever with renal syndrome [1,2]. These small mammals frequently harbor a multitude of ectoparasites such as fleas, sucking lice, gamasid mites, chigger mites, and occasionally ticks [3,4]. Rodents are of paramount importance among small mammal hosts [5]. Ectoparasites found on rodents and other small mammals act as vectors for a variety of zoonotic diseases, with fleas transmitting plague and murine typhus, ticks transmitting encephalitis and Lyme disease, chigger mites transmitting scrub typhus, and gamasid mites transmitting rickettsialpox. Gamasid mites may also serve as potential vectors for hemorrhagic fever with renal syndrome (HFRS) [6–12]. Although ectoparasites are of medical importance, to date, few sources of literature have provided a systematic and comprehensive protocol for on-site monitoring and specimen preparation of ectoparasites. Based on relevant literature and our research experience on ectoparasites, this protocol systematically describes methods for on-site monitoring and specimen-making of ectoparasites.
Small mammals in this protocol refer to rodents (Order Rodentia) and other sympatric small mammals found in the same habitats. These include insectivores (Order Eulipotyphla or Insectivora), tree shrews (Order Scandentia), and pikas (Order Lagomorpha), excluding flying bats (Order Chiroptera). Small mammals serve as hosts of ectoparasites.
Fleas and sucking lice, two groups of ectoparasites on small mammals, belong to the class Insecta from the phylum Arthropoda [13,14], and their collection and specimen preparation methodologies are similar. Gamasid mites and chigger mites, two tiny categories of arthropods, belong to the subclass Acari, class Arachnida of Arthropoda [15,16], and their collection and specimen preparation methodologies are also similar. Chigger mites are unique among Acari, with only the larval stage acting as ectoparasites; other stages are free-living [17–20]. Ticks predominantly parasitize large and medium-sized mammals, but occasionally, a few tick larvae and nymphs can be found parasitizing rodents and other small mammals [21-23]. Although ticks belong to the subclass Acari of Arachnida [24], their collection and specimen preparation methods differ from those of gamasid mites and chigger mites due to their relatively larger size.
Materials and reagents
Reagents
Cresol solution (Shanghai Hengyuan Biochemical Reagent, catalog number: 7784-24-9)
Potassium aluminum sulfate (Shanghai Hengyuan Biochemical Reagent, catalog number: 7784-24-9)
Arsenic trioxide (Yunnan Tin Co, catalog number: 1327-53-3)
Camphor powder (Shanghai Anpu Experimental Technology, catalog number: 464-49-3)
Xylene (Shanghai Aladdin Biochemical Technology, catalog number: 1330-20-7)
Distilled water
Canadian balsam (Shanghai Macklin Biochemical Technology, catalog number: 8007-47-4), neutral balsam (Shanghai Macklin Biochemical Technology, catalog number: 96949-21-2), or fir balsam (Aibixin Biotechnology, catalog number: 8016-42-0)
Pomegranate oil (Xi’an Rongbai Biological Technology, catalog number: 84961-57-9) or clove oil (Jiangxi Baicao Pharmaceutical, catalog number: 8000-34-8)
Arabic gum (Shanghai Yiji Industrial, catalog number: 9000-01-5)
Formaldehyde (Beijing Qinling Pharmaceutical Technology, catalog number: 79083-29-7)
Glycerin (Shanghai Aladdin Biochemical Technology, catalog number 56-81-5)
10% sodium hydroxide (Shanghai Macklin Biochemical Technology, catalog number 1310-73-2) or potassium hydroxide (Shanghai Aladdin Biochemical Technology, catalog number 1310-58-3) solution
5%–10% cresol soap solution (Lysol) (see Recipes)
Alum arsenic preservative (see Recipes)
Hoyer’s solution or Berlese’s solution (see Recipes)
Different concentrations of ethanol (30%, 50%, 70%, 80%, 90%, 95% and 100% anhydrous ethanol)
The different concentrations of ethanol can be prepared by diluting anhydrous ethanol with distilled water. Anhydrous ethanol is a kind of very commonly used reagent with a wide choice of catalog numbers and manufacturers. We use anhydrous ethanol from Wuhan Jisi Instruments & Equipment (catalog number: 64-17-5).
Recipes
5%–10% cresol soap solution (Lysol)
5 mL of cresol solution + 95 mL of distilled water for making 5% cresol soap solution.
10 mL of cresol solution + 90 mL of distilled water for making 10% cresol soap solution.
In a beaker, mix the cresol and water and shake or stir until the cresol is completely dissolved in the water.
Alum arsenic preservative
Potassium aluminum sulfate (powder, accounting for 40% or 70%) + arsenic trioxide (powder, 20%) + camphor powder (40% or 10%). Mix them together.
Hoyer’s solution or Berlese’s solution
Hoyer’s solution (or Hoyer’s medium)
50 mL of distilled water, 30 g of Arabic gum, 200 g of formaldehyde, 20 mL of glycerin.
Berlese’s solution (or Berlese medium)
20 mL of distilled water, 15 g of Arabic gum, 160 g of formaldehyde, 20 mL of glycerin.
Laboratory supplies
Collection and specimen preparation of small mammal hosts
Fieldwork equipment (field tent, backpack, raincoat, non-slip shoes, flashlight)
Disinfection and protection materials: 70% or 75% ethanol (Wuhan Jisi Instruments & Equipment, catalog number: 64-17-5), protective clothing and hat, medical latex gloves, mask
Trapping materials
Rat cage (18 cm × 12 cm × 9 cm, Jiangxi Guixi Li’s Rat Trap Equipment Co., Ltd.)
Food bait (peanuts, corn, cooked meat, fried dough sticks, steamed buns)
Disposable white cloth bag
Recording materials (pen, marker, pencil, collection information record book)
Specimen preparation materials for small mammals [lancet, surgical scissors, tweezers, steel ruler or tape measure, electronic scale, degreased cotton, bamboo sticks, wooden sticks, suture needles, specimen labels, beakers, alcohol lamps (for boiling small animal skulls), ether, alum arsenic preservative]
Host tissue sample collection and preservation materials: Cryopreservation tubes and 95%–100% alcohol
Collection and fixation of ectoparasites
White square plate (large size: 565 mm × 400 mm, small size: 385 mm × 285 mm)
Lidded centrifuge tubes
Ophthalmic forceps
70% or 75% ethanol
Marking pens
Specimen-making of flea and sucking lice
Glass slides
Coverslips
Baking plate or baking rack
Specimen box
Marker pen
Label paper
Specimen-making of gamasid mites and chigger mites
Petri dish
Brush
Slide
Coverslip
Marker pen
Label paper
Specimen-making of ticks
Wide-mouth bottle
Cover glass
Sealing wax
Equipment
Stereomicroscope (Leica, model: DM3000)
Baking oven (Shanghai Yiheng Scientific Instrument, model: DHG-9420A)
Procedure
文章信息
稿件历史记录
提交日期: Mar 17, 2024
接收日期: Sep 2, 2024
在线发布日期: Oct 22, 2024
出版日期: Nov 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Yin, P., Guo, X., Song, W., Dong, W., Lv, Y. and Jin, D. (2024). The on-Site Monitoring and Specimen-Making of Ectoparasites on Rodents and Other Small Mammals. Bio-protocol 14(21): e5104. DOI: 10.21769/BioProtoc.5104.
分类
环境生物学 > 寄生生物
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










