- EN - English
- CN - 中文
An In Vitro Model of Murine Osteoclast-Mediated Bone Resorption
小鼠破骨细胞介导的骨吸收体外模型
发布: 2024年11月05日第14卷第21期 DOI: 10.21769/BioProtoc.5100 浏览次数: 2203
评审: Valérian DORMOYJaira Ferreira de VasconcellosNona Farbehi
Abstract
Osteoclasts are terminally differentiated multinucleated giant cells that mediate bone resorption and regulate skeletal homeostasis under physiological and pathological states. Excessive osteoclast activity will give rise to enhanced bone resorption, being responsible for a wide range of metabolic skeletal diseases, ranging from osteoporosis and rheumatoid arthritis to tumor-induced osteolysis. Therefore, the construction of in vitro models of osteoclast-mediated bone resorption is helpful to better understand the functional status of osteoclasts under (patho)physiological conditions. Notably, it is essential to provide an in vivo–relevant bone substrate that induces osteoclasts to generate authentic resorption lacunae and excavate bone. Here, we summarize the experimental design of a reproducible and cost-effective method, which is suitable for evaluating the regulatory mechanisms and influence of molecular agonists and antagonists as well as therapeutics on osteoclast-mediated bone-resorbing activity.
Key features
• Experiments are performed using bovine cortical bone slices to simulate bone substrate resorption by murine osteoclasts in vivo.
• The method allows for quantification of bone resorption in vitro.
• The method is suitable for evaluating the regulatory mechanisms that control osteoclast-mediated bone-resorbing activity.
Keywords: Osteoclast (破骨细胞)Graphical overview
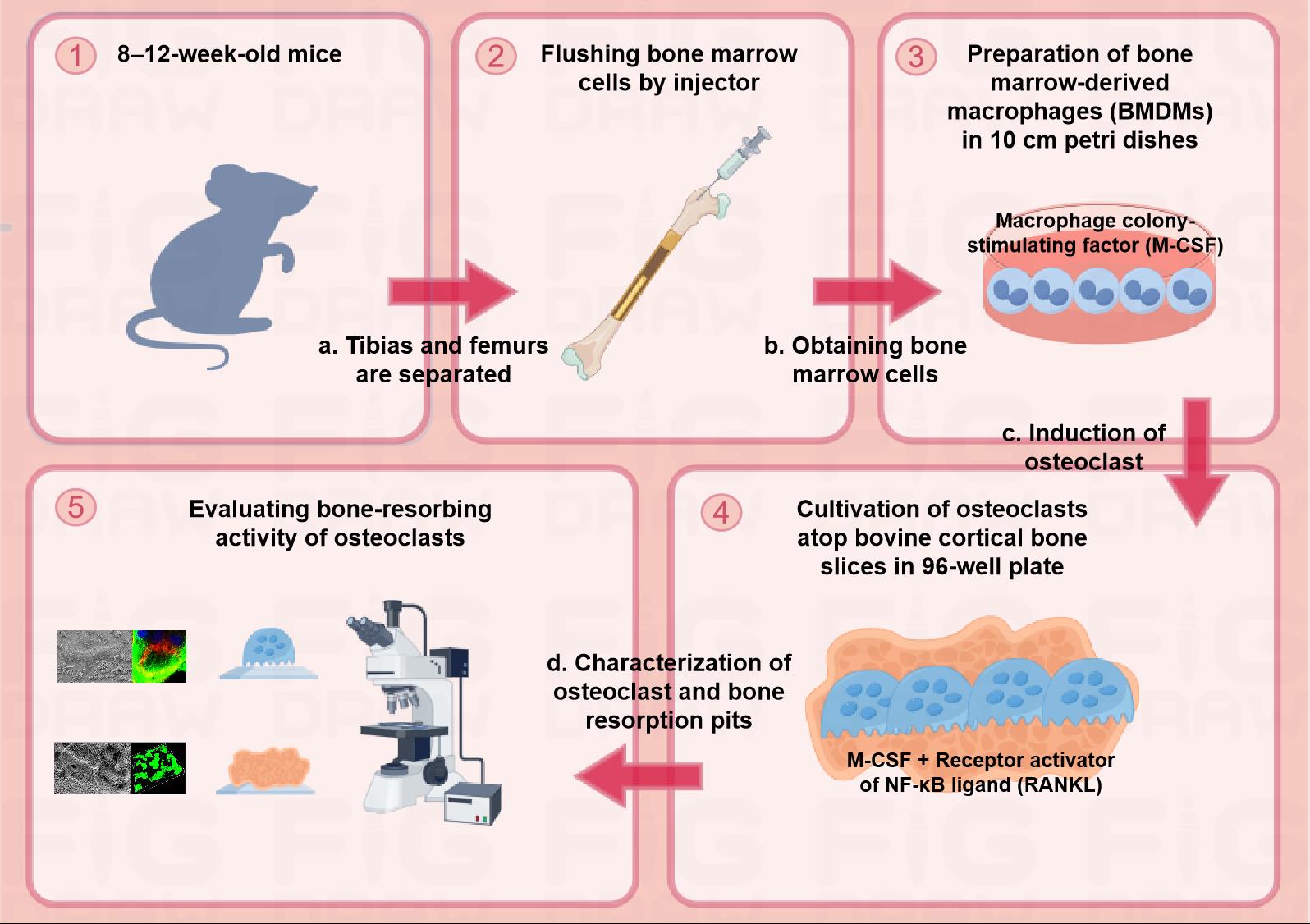
Schematic diagram of the protocol for assessing osteoclast-mediated bone-resorbing activity in vitro. Step 1: Euthanizing 8–12-week-old male mice to separate tibias and femurs. Step 2: Flushing bone marrow cells by injector and obtaining the cells. Step 3: Preparation of BMDMs with complete medium containing 20 ng/mL M-CSF in 10 cm Petri dishes. Step 4: Cultivation of osteoclasts with complete medium containing 20 ng/mL M-CSF and 30 ng/mL RANKL atop bovine cortical bone slices in a 96-well plate. Step 5: Evaluating bone-resorbing activity of osteoclasts.
Background
Osteoclasts are exclusive and specialized multinucleated bone-resorbing cells derived from both embryonic and hematopoietic stem cell precursors of erythromyeloid and myeloid lineages [1]. An imbalance of skeletal remodeling as a consequence of increased osteoclast-mediated bone resorption is responsible for a wide range of metabolic skeletal diseases, ranging from osteoporosis and rheumatoid arthritis to tumor-induced osteolysis. Therefore, the construction of in vitro osteoclast-mediated bone resorption models is helpful to better characterize the functional status of osteoclasts under both physiological and pathological conditions.
The transition of cells of the monocyte/macrophage lineage into osteoclasts in vitro requires two essential cytokines—macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-kB ligand (RANKL). The presence of M-CSF promotes the proliferation of osteoclast precursor cells, and RANKL further induces precursor cells to express an osteoclast phenotype [2]. Mature osteoclasts at the bone surfaces undergo a polarization process marked by extensive morphologic changes, including the formation of an actin ring at their basal surface, termed the sealing zone. The sealing zone surrounds a differentiated and intricately folded region of the plasma membrane called the ruffled border, where protons, chloride ions, and proteases are secreted into the underlying resorption lacunae where bone demineralization and extracellular matrix degradation proceed [3]. As the organic phase of bone is dominated by type I collagen, the proteolytic release of C-terminal telopeptide fragments of type collagen (CTX-1) is a characteristic biochemical marker of osteoclast-mediated bone resorption [4]. When cultured on bone or dentin substrate, osteoclasts assemble resorption lacunae, thereby generating resorption pit structures similar to those formed during bone resorption in vivo [5]. Notably, bovine cortical bone slices are superior to synthetic materials or dentine-derived matrix as they better mimic bone substrates encountered in vivo [6]. When combined with an appropriate detection assay, osteoclast-mediated bone-resorbing activity can be quantified under a variety of experimental conditions [7,8].
Here, we describe a step-by-step protocol for in vitro bone-resorbing activity using an osteoclast-cortical bone slice co-culture system. This protocol consists of three main steps: mouse bone marrow–derived macrophage (BMDM) preparation, osteoclast induction and characterization, and bone resorption pit quantification. This method can be universally utilized to evaluate the bone-resorbing activity of osteoclasts in response to both agonists and antagonists.
Materials and reagents
Biological materials
8–12-week-old male C57BL/6J mice (The Jackson Laboratory, catalog number: 000664)
Bovine cortical bone slices (Immunodiagnostic Systems, catalog number: DT-1BON1000-96)
Reagents
Alpha minimum essential medium (α-MEM) (Hyclone, Cytiva, catalog number: SH30265.01)
Fetal bovine serum (FBS) (Sigma-Aldrich, catalog number: F0193)
Penicillin-streptomycin solution (Hyclone, Cytiva, catalog number: SV30010)
Red blood cell lysing buffer (Biosharp, catalog number: BL504A)
Phosphate buffered saline (PBS) (Servicebio, catalog number: G0002)
Ethylenediaminetetraacetic acid (EDTA) solution, 0.5 M (Sigma-Aldrich, catalog number: 03690)
Recombinant murine M-CSF (R&D Systems, catalog number: 416-ML)
Recombinant murine RANKL (R&D Systems, catalog number: 462-TEC)
Bovine serum albumin (Sigma-Aldrich, catalog number: A1933)
Paraformaldehyde powder (Sigma-Aldrich, catalog number: 158127)
Glutaraldehyde solution, 50% (Sigma-Aldrich, catalog number: 49629)
Tartrate-resistant acid phosphatase (TRAP) stain kit (Sigma-Aldrich, catalog number: 387A)
Triton X-100 solution, 10% (Sigma-Aldrich, catalog number: 49629)
Phalloidin-tetramethylrhodamine B isothiocyanate (Sigma-Aldrich, catalog number: P1951)
α-Tubulin mouse mAb (Cell Signaling Technology, catalog number: 3873)
Donkey anti-rat Alexa 488-conjugated secondary antibodies (Invitrogen Molecular Probes, catalog number: A-21202)
Sodium hydroxide (NaOH) powder (Sigma-Aldrich, catalog number: 655104)
Peroxidase-conjugated wheat germ agglutinin (WGA) (Sigma-Aldrich, catalog number: L3892)
Diaminobenzidine (DAB) tablets (Sigma-Aldrich, catalog number: D4418)
FITC conjugate WGA (Sigma-Aldrich, catalog number: L4895)
CrossLaps ELISA kit for quantifying CTX-1 release (Immunodiagnostic Systems, catalog number: AC-07F1)
Solutions
5 mM EDTA solution (see Recipes)
0.1% Bovine serum albumin solution (see Recipes)
Murine recombinant M-CSF solution (see Recipes)
Murine recombinant RANKL solution (see Recipes)
4% Paraformaldehyde solution (see Recipes)
2.5% Glutaraldehyde solution (see Recipes)
NaOH solution (see Recipes)
0.1% Triton X-100 solution (see Recipes)
Peroxidase-conjugated WGA solution (see Recipes)
FITC-conjugated WGA solution (see Recipes)
Recipes
5 mM EDTA solution
Reagent Final concentration Quantity or Volume Recommended storage condition Recommended storage time 0.5 M EDTA solution 5 mM 0.5 mL Room temperature Sterile PBS NA 49.5 mL Room temperature Final volume 50 mL 4 1 week 0.1% Bovine serum albumin solution
Reagent Final concentration Quantity or Volume Recommended storage condition Recommended storage time Bovine serum albumin 0.1% 0.02 g 4 Double-distilled water NA 20 mL Room temperature Final volume 20 mL -20 1 month Murine rM-CSF solution
Reagent Final concentration Quantity or Volume Recommended storage condition Recommended storage time Murine rM-CSF 50 μg/mL 50 μg -80 Sterile PBS containing 0.1% bovine serum albumin NA 1 mL -20 Final volume 1 mL -80 1 year Murine rRANKL solution
Reagent Final concentration Quantity or Volume Recommended storage condition Recommended storage time Murine rRANKL 50 μg/mL 50 μg -80 Sterile PBS containing 0.1% bovine serum albumin NA 1 mL -20 Final volume 1 mL -80 1 year 4% Paraformaldehyde solution
Reagent Final concentration Quantity or Volume Recommended storage condition Recommended storage time Paraformaldehyde powder 4% (w/v) 0.4 g -4 Sterile PBS NA 10 mL Room temperature Final volume 10 mL -4 1 week 2.5% Glutaraldehyde solution
Reagent Final concentration Quantity or Volume Recommended storage condition Recommended storage time 50% glutaraldehyde solution 2.5% (v/v) 0.5 mL Room temperature Sterile PBS NA 9.5 mL Room temperature Final volume 10 mL -4 1 week NaOH solution
Reagent Final concentration Quantity or Volume Recommended storage condition Recommended storage time NaOH powder 0.5 N 1 g Room temperature Double-distilled water NA 50 mL Room temperature Final volume 50 mL -4 1 month 0.1% Triton X-100 solution
Reagent Final concentration Quantity or Volume Recommended storage condition Recommended storage time 10% Triton X-100 solution 0.1% 0.01 mL Room temperature Double-distilled water NA 9.99 mL Room temperature Final volume 10 mL -4 1 month Peroxidase-conjugated WGA solution
Reagent Final concentration Quantity or Volume Recommended storage condition Recommended storage time Peroxidase-conjugated WGA 2 mg/mL 1 mg -20 Sterile PBS NA 0.5 mL Room temperature Final volume 0.5 mL -20 1 year FITC-conjugated WGA solution
Reagent Final concentration Quantity or Volume Recommended storage condition Recommended storage time FITC-conjugated WGA 2 mg/mL 1 mg -20 Sterile PBS NA 0.5 mL Room temperature Final volume 0.5 mL -20 1 year
Laboratory supplies
10 mL syringe with 25 G needle
Sterile absorbent gauze
70 µm cell strainer (Corning, catalog number: 431751)
Pipette
15 mL conical tube (Nest, catalog number: 601001)
50 mL conical tube (Nest, catalog number: 601002)
10 × 10 cm Petri dish with cover (Nest, catalog number: 752001)
Cell lifter (Corning, catalog number: CLS3008)
96-well cell culture plates (Nest, catalog number: 713011)
Equipment
Dissecting tweezers and scissors (Autoclaved)
Cell counter (Thermo Fisher Scientific Inc, model: Countess 3)
Cell culture incubator (37 °C, 5% CO2)
Centrifuge (Eppendorf)
Inverted microscope (Olympus)
Confocal laser scanning microscope (CLSM) (Nikon A1)
Critical point drying (Balzers Union)
Au/Pg sputtered (Polaron Equipment Ltd, model: E-5100)
Field emission scanning electron microscopy (FE-SEM) (AMRAY 1910)
Software and datasets
ImageJ is a Java-based (runs on Mac OS X, Linux, and Windows) freeware available for download at: http://rsb.info.nih.gov/ij/
GraphPad Prism 7
Procedure
文章信息
稿件历史记录
提交日期: Jun 20, 2024
接收日期: Sep 3, 2024
在线发布日期: Oct 13, 2024
出版日期: Nov 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Sun, X., Wang, Z., Tang, Y., Weiss, S. J. and Zhu, L. (2024). An In Vitro Model of Murine Osteoclast-Mediated Bone Resorption. Bio-protocol 14(21): e5100. DOI: 10.21769/BioProtoc.5100.
- Zhu, L., Tang, Y., Li, X. Y., Kerk, S. A., Lyssiotis, C. A., Sun, X., Wang, Z., Cho, J. S., Ma, J., Weiss, S. J., et al. (2023). Proteolytic regulation of a galectin-3/Lrp1 axis controls osteoclast-mediated bone resorption. J Cell Biol. 222(4): e202206121.
分类
发育生物学 > 细胞生长和命运决定 > 分化
细胞生物学 > 细胞分离和培养 > 细胞分化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link