- EN - English
- CN - 中文
Optimizing Transmembrane Protein Assemblies in Nanodiscs for Structural Studies: A Comprehensive Manual
用于结构研究的纳米盘跨膜蛋白组装优化:综合手册
(*contributed equally to this work) 发布: 2024年11月05日第14卷第21期 DOI: 10.21769/BioProtoc.5099 浏览次数: 3070
评审: Beatrice LiAnonymous reviewer(s)
Abstract
Membrane protein structures offer a more accurate basis for understanding their functional correlates when derived from full-length proteins in their native lipid environment. Producing such samples has been a primary challenge in the field. Here, we present robust, step-by-step biochemical and biophysical protocols for generating monodisperse assemblies of full-length transmembrane proteins within lipidic environments. These protocols are particularly tailored for cases where the size and molecular weight of the proteins align closely with those of the lipid islands (nanodiscs). While designed for single-span bitopic membrane proteins, these protocols can be easily extended to proteins with multiple transmembrane domains. The insights presented have broad implications across diverse fields, including biophysics, structural biology, and cryogenic electron microscopy (cryo-EM) studies.
Key features
• Overview of the sample preparation steps from protein expression and purification and reconstitution of membrane proteins in nanodiscs, as well as biobeads and lipids preparation.
• Focus on single-span bitopic transmembrane proteins.
• Includes protocols for validation procedures via characterization using biochemical, biophysical, and computational techniques.
• Guide for cryogenic electron microscopy data acquisition from vitrification to image processing.
Keywords: Nanodisc (纳米盘)Graphical overview
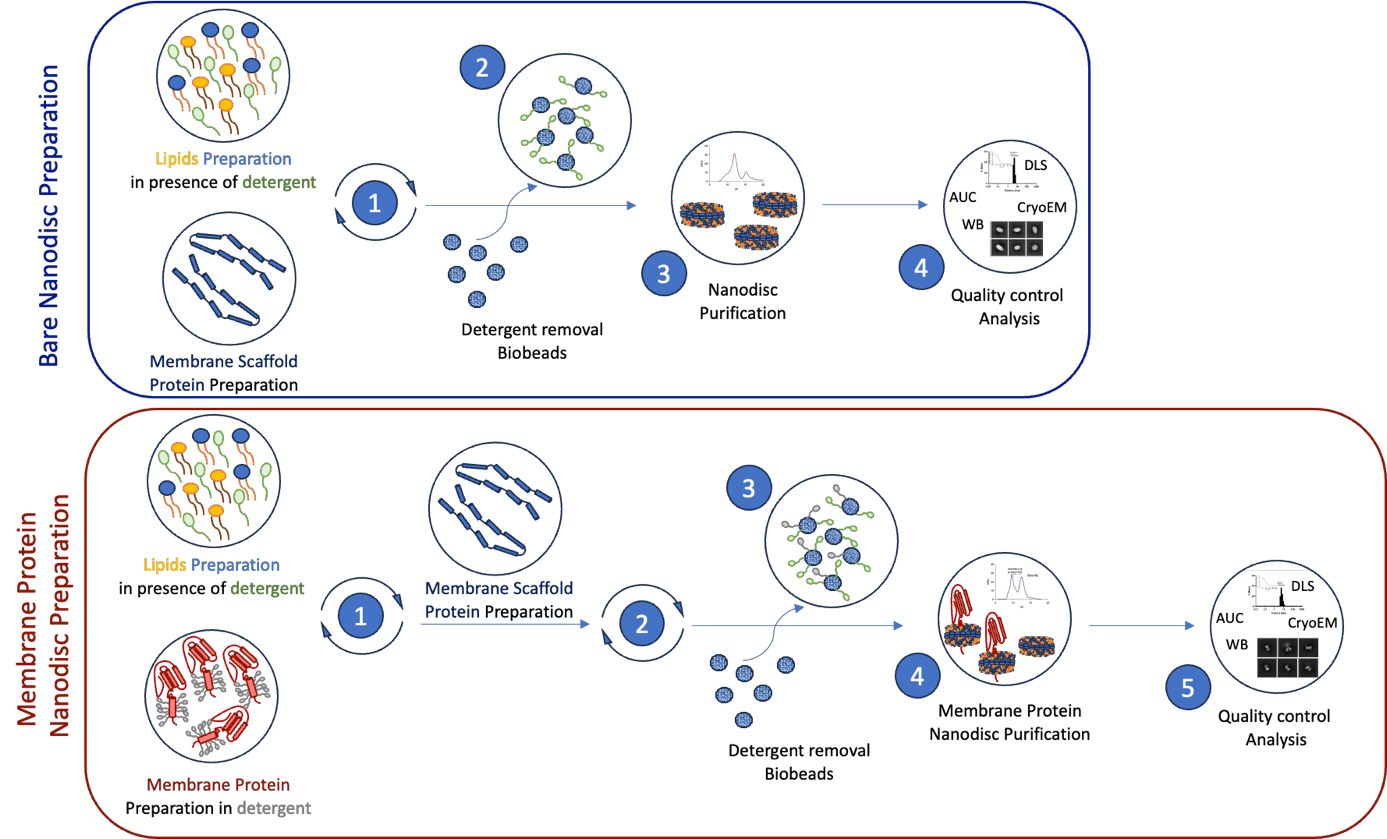
Schematic representation of the workflow for membrane protein reconstitution in nanodiscs. The upper blue box illustrates the key steps involved in preparing bare nanodiscs from lipid and membrane scaffold protein preparations (1), removal of detergent (2), and purification of nanodiscs (3). The quality of the samples is verified through various biochemical and imaging approaches (4). The lower red box outlines the step-by-step membrane protein-nanodiscs reconstitution process. Detergent-solubilized and purified membrane protein samples are mixed with the chosen lipid sample (1), followed by the addition of membrane scaffold proteins (2). Dialysis in the presence of bio-beads allows for detergent removal (3), leading to the reconstitution of membrane protein-nanodiscs complexes, followed by size exclusion chromatography purification (4). Finally, we present various biophysical and screening methods to assess the quality and homogeneity of the samples before structural analysis (5).
Background
Transmembrane proteins play a pivotal role in the interactions of cells with their environment. Their significance in pharmacology is underscored by their targeting over 50% of available drugs [1]. A more profound comprehension of the structure and function of these proteins can greatly enhance our understanding of disease development and the creation of effective medications. However, exploring the structure of membrane proteins poses a significant challenge due to the hydrophobic nature of their transmembrane domains, which play crucial functional roles. A comprehensive study necessitates examining these proteins in their entirety, including their transmembrane domains, within stable lipid environments close to their native conditions in the cell. Meeting these prerequisites calls for the development of specialized biochemical tools tailored for the study of transmembrane proteins.
One such tool is the nanodisc (ND), a nanoscale disc-shaped lipid bilayer encircled by a belt formed by two membrane scaffold proteins (MSPs). Nanodiscs provide numerous advantages compared to traditional solubilization methods, including enhanced stability, uniformity, and the ability to explore membrane proteins in their native lipid environment. Since the pioneering work of Sligar and colleagues, MSPs have revolutionized various fields including structural biology and biophysics [2–6]. MSP nanodiscs maintain the solubilization of full-length transmembrane proteins, preventing detergent-induced denaturation. When the size and molecular weight (MW) of the transmembrane protein significantly exceed that of the NDs or when the transmembrane domain size approaches that of the nanodisc and displaces a large fraction of the lipids, the system serves as a potent tool for structural biology investigations. For example, NDs have been instrumental in exploring the structure and function of membrane proteins such as G protein–coupled receptors, ion channels, and transporters [7–9]. However, this scenario changes when the NDs and the transmembrane protein share a similar MW range and the transmembrane displaces only a small fraction of the nanodisc lipids, for example, single-spanning membrane receptors such as integrin. Under the premises where the nanodiscs make a substantial contribution to the overall structural analysis, imperfections in the nanodisc preparations directly translate into lower resolution in cryo-EM structural studies.
Here, we detail comprehensive and reliable biochemical and biophysical protocols that enable the structural examination of transmembrane proteins, particularly when their size and MW are similar to the nanodiscs, and they occupy only a small fraction of the nanodisc. Our focus is a thorough manual for researchers concerning the use of MSPs and MSP-based NDs in the structural and biophysical examination of single-pass alpha-helix transmembrane proteins, often referred to as bitopic transmembrane proteins. While this publication is tailored for bitopic transmembrane proteins, the principles outlined can be applied to the study of proteins with multiple transmembrane domains.
Materials and reagents
Biological materials
E. coli BL21(DE3) chemically competent cells (Sigma-Aldrich, catalog number: CMC0014)
Expression vectors of membrane scaffold proteins (MSP) from Addgene: MSP1D1 (Addgene, catalog number: 20061), MSP1E3D1 (Addgene, catalog number: 20066), and MSP2N2 (Addgene, catalog number: 29520) for 9, 12, and 15 nm nanodiscs, respectively
Purified and monodisperse target membrane protein of your interest. Here, we use ACE2 protein as an example of single-pass transmembrane protein, which was purified in 50 mM HEPES pH 8, 250 mM NaCl, 0.5% DDM, and 2.5 mM desthiobiotin (adapted from Yan et al. [10])
Reagents
POPC lipids in chloroform, 25 mg/mL (Avanti Polar Lipids, catalog number: 850457C)
2× YT medium (Sigma-Aldrich, catalog number: Y2377)
94 mm Petri dish (Grenier Bio-One, catalog number: 633180) containing 20 mL of LB agar media
Kanamycin at 50 µg/mL for selecting bacteria carrying vectors of MSPs (Sigma-Aldrich, catalog number: K0254)
Trizma base (purity ≥ 99.7%) (Sigma-Aldrich, catalog number: 93350)
Hydrochloric acid 37% vol/vol (HCl) (Sigma-Aldrich, catalog number: 258148)
Sodium chloride (NaCl); purity ≥ 99% (Sigma-Aldrich, catalog number: 793566)
Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: 03677)
Imidazole (purity ≥ 99.5%) (Sigma-Aldrich, catalog number: 56750)
Ethanol absolute (99.8% analytical reagent grade) (Fisher Chemical, catalog number: E/0650DF/15)
CAUTION: Ethanol is harmful if swallowed, inhaled, or by skin absorption. Wear appropriate protective glasses, gloves, and lab coat. Handle under a chemical hood.
MilliQ H2O
Buffer components required by the target membrane protein of your interest
Methanol (Sigma-Aldrich, catalog number: 322415)
CAUTION: Methanol is toxic if swallowed, inhaled, or in contact with skin. Wear appropriate protective glasses, gloves, and lab coat. Handle under a chemical hood.
Chloroform (99.5%) (Sigma-Aldrich, catalog number: C2432)
CAUTION: Chloroform is toxic if swallowed or inhaled. Always wear googles, gloves, and lab coat. Carry operations under a chemical hood.
Sodium cholate (NaCholate) (Sigma-Aldrich, catalog number: C6445)
Triton X-100 (Sigma-Aldrich, catalog number: T9284)
PMSF (Roche, catalog number: 10837091001)
Anti-proteases cocktail (Roche, catalog number: 11836170001)
Benzonase® (Sigma-Aldrich, catalog number: 1016970001)
Lysozyme (Sigma-Aldrich, catalog number: 1052810500)
Isopropyl b-D-1-thiogalactopyranoside (IPTG) (Sigma-Aldrich, catalog number: I6758)
Bio-Beads SM-2 adsorbents (Bio-Rad, catalog number: 1528920)
Antibody for His-tag detection (Thermo Scientific, catalog number: 15547466)
4–15% Mini-PROTEAN® TGXTM precast protein gels (Bio-Rad, catalog number: 4561086)
Liquid nitrogen (pure from Air Liquide ALPHAGAZ)
CAUTION: Liquid nitrogen can cause severe burns. Always wear required personal safety equipment such as safety glasses, lab coat, and insulated gloves when handling.
Nitrogen (N2) gas (purity ≥ 99,999%) (Air Liquide ALPHAGAZ)
Ethane gas (pure from Air Liquide ALPHAGAZ)
CAUTION: Ethane gas is extremely flammable. Store in tightly closed containers in a cool, well-ventilated area.
Solutions
Lipid solubilization buffer (see Recipes)
Size exclusion chromatography buffer (see Recipes)
Lysis buffer (see Recipes)
MSP wash buffer (see Recipes)
Elution buffer (see Recipes)
Recipes
Lipid solubilization buffer
Reagent Final concentration Quantity or Volume Trizma pH 7.4 20 mM 1.21 g NaCl 100 mM 2.92 g EDTA pH 7.4 0.5 mM 93.06 mg NaCholate 70 mM 15.07 g H2O n/a Final volume to 500 mL Note: Small increments of HCl are used to bring the solution to pH 7.4.
Size exclusion chromatography buffer
Reagent Final concentration Quantity or Volume Trizma pH 7.4 20 mM 1.21 g NaCl 100 mM 2.92 g EDTA pH 7.4 0.5 mM 93.06 mg H2O n/a Final volume to 500 mL Note: Small increments of HCl are used to bring the solution to pH 7.4.
Lysis buffer
Reagent Final concentration Quantity or Volume Trizma pH 8.0 50 mM 454.28 mg NaCl 300 mM 1.31 g Triton X-100 1% 750 µL PMSF 1 mM 13.06 mg Tablet anti-protease cocktail 1 tablet Benzonase® 250 unit 1 µL Lysozyme 0.7 mg per mL of lysis buffer 52.5 mg H2O n/a Final volume to 75 mL per L of E. coli culture Note: Small increments of HCl are used to bring the solution to pH 8.0. Initially, a larger volume of solution containing only Trizma, NaCl, and Triton X-100 can be made. On the day of the experiment, use 75 mL of this solution and supplement with PMSF, anti-protease cocktail, benzonase, and lysozyme to perform bacterial lysis.
MSP wash buffer
Reagent Final concentration Quantity or Volume Trizma pH 8.0 50 mM 3.03 g NaCl 300 mM 8.77 g H2O n/a Final volume to 500 mL Note: Small increments of HCl are used to bring the solution to pH 8.0.
Elution buffer
Reagent Final concentration Quantity or Volume Trizma pH 8.0 50 mM 3.03 g NaCl 300 mM 8.77 g Imidazole 500 mM 17.02 g H2O n/a Final volume to 500 mL Note: Small increments of HCl are used to bring the solution to pH 8.0.
Laboratory supplies
Cryo-EM grids such as Quantifoil 1.2/1.3 400 mesh copper (Quantifoil, catalog number: N1-C14nCu40-01)
Filter paper for VitroBot (Grade 595, 50 mm, AA00420S) (Fisher Scientific, catalog number: 09-924-170)
Hamilton syringe 500 Ul (Merck, catalog number: 24523)
Spectra/Por 3 dialysis tubing, 3.5 kD MWCO (Repligen, catalog number: 13272)
Costar® Spin-X® centrifuge tube filters, 0.22 µm pore CA membrane, nonsterile, 100/case (Corning, catalog number: 8161)
Parafilm (Bemis, catalog number: PM996)
10 mL glass tubes (Kimble Chase, catalog number: A407511500611)
His-trap column 5 mL affinity chromatography (Cytiva, catalog number: 17524802 or similar)
Size-exclusion Superdex 200 increase 24 mL chromatography column (Cytiva, catalog number: 28990944 or similar)
Amicon concentrators cutoff 50 kDa (Millipore, catalog number: UFC505096)
InstantBlue® Coomassie protein stain (Abcam, catalog number: ab119211)
Storage dewar for EM grids (Worthington LD Series Liquid Nitrogen Dewars, catalog number: F9143-1EA)
Laboratory glassware and plasticware for preparation, storage, and handling of reagents and samples
NalgeneTM single-use PETG Erlenmeyer flasks with baffled bottom 2 L (Nalgene, catalog number: 4113-2000)
PES (Polyethersulfone) syringe filters (Thermo Scientific, catalog number: CH2213-PES)
Micropipette tips and plastic pipettes cut at about 1/3 from the tip to be used when manipulating Bio-Beads
Equipment
Spectrophotometer NanoDrop (Thermo Scientific, or similar) for OD600 bacterial density and absorbance at 280 (A280) protein concentration measurements
Incubator with temperature control for Erlenmeyer flasks
Rotator TR Series TR-200 (Stuart/Cole-Palmer, model: TR-200)
Mini-PROTEAN Tetra Vertical electrophoresis cell (Bio-Rad, catalog number: 1658004)
Batch sonicator (Fisher Scientific, catalog number: FB15046)
pH meter (Thermo Scientific, catalog number: STARA1115) and calibration reagents (Thermo Scientific, catalog number: 910199)
Dry bath with temperature control such as Eppendorf ThermoMixer F2.0 (Eppendorf, catalog number: 5387000013)
FPLC Equipment, Akta System (Cytiva) or similar
Western blot device iBind and accessories (Thermo Scientific, catalog number: SLF2000)
Imaging system for SDS-Page and western blot (Bio-Rad, model: Gel DocTM EZ System)
Lab bench centrifugation equipment such as Eppendorf centrifuge 5424 R (Eppendorf, model: 5424 R)
Bench microcentrifuge such as Scientific D1008 Palm microcentrifuge (DLAB, catalog number: 9031001012)
Sample storage equipment at -80 °C, -20 °C, and 4 °C
Chemical hood
VitroBot Mark IV (or similar) (Thermo Scientific)
Software and datasets
Chromatography Akta software: Unicorn 6.3 or later version, Cytiva
Imaging software for western blot and SDS page: Image Lab 6.0 or later version, Bio-Rad
DLS software: DYNAMICS software 7.9 or later version, Waters
Data Acquisition software for single particle analysis: EPU 3.3.0.5176REL or later SerialEM 3 or higher version
Data analysis software for single particle analysis: CryoSPARC version 4.3.0 or higher, Relion 4 or equivalent
Procedure
文章信息
稿件历史记录
提交日期: May 29, 2024
接收日期: Sep 1, 2024
在线发布日期: Sep 28, 2024
出版日期: Nov 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Vilela, F., Sauvanet, C., Bezault, A., Volkmann, N. and Hanein, D. (2024). Optimizing Transmembrane Protein Assemblies in Nanodiscs for Structural Studies: A Comprehensive Manual. Bio-protocol 14(21): e5099. DOI: 10.21769/BioProtoc.5099.
分类
生物物理学 > 显微技术 > 低温显微镜技术
生物化学 > 蛋白质 > 结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link