- EN - English
- CN - 中文
Fluorescence Lifetime-Assisted Probing of Protein Aggregation with sub-Organellar Resolution
通过荧光寿命技术辅助探测具有亚细胞器分辨率的蛋白质聚集
发布: 2024年10月05日第14卷第19期 DOI: 10.21769/BioProtoc.5080 浏览次数: 2003
评审: Elena A. OstrakhovitchJose Martinez HernandezAnonymous reviewer(s)
Abstract
Protein misfolding fuels multiple neurodegenerative diseases, but existing techniques lack the resolution to pinpoint the location and physical properties of aggregates within living cells. Our protocol describes high-resolution confocal and fluorescent lifetime microscopy (Fast 3D FLIM) of an aggregation probing system. This system involves a metastable HaloTag protein (HT-aggr) labeled with P1 solvatochromic fluorophore, which can be targeted to subcellular compartments. This strategy allows to distinguish between aggregated and folded probe species, since P1 fluorophore changes its lifetime depending on the hydrophobicity of its microenvironment. The probe is not fluorescence intensity-dependent, overcoming issues related to intensity-based measurements of labeled proteins, such as control of probe quantity due to differences in expression or photobleaching of a proportion of the fluorophore population. Our approach reports on the performance of the machinery dealing with aggregation-prone substrates and thus opens doors to studying proteostasis and its role in neurodegenerative diseases.
Key features
• Aggregation state: Tracks aggregate formation and disaggregation with pulse-chase experiments
• Sub-organellar resolution: Pinpoints and allows control of aggregate location within the cell, exceeding traditional techniques
• Quantitative analysis: Measures aggregate load through image analysis
• Methodology:
Metastable HaloTag variant labeling with a solvatochromic small-molecule reporter ligand
High-resolution confocal microscopy coupled with FLIM for aggregate identification and localization
Image analysis for aggregate quantification and distribution within the ER
Pulse-chase experiments to track aggregates
Graphical overview
Background
Protein misfolding and aggregation are hallmarks of numerous neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis (ALS) [1]. Unraveling how the protein folding and quality control system handles aggregation-prone substrates is key to understanding why protein folding mechanisms fail, leading to age-associated aggregate accumulation in disease [2].
Proteostasis, the intricate network governing protein homeostasis within cells, relies on the coordinated efforts of various organelles and molecular machinery. Each cellular compartment contributes uniquely to the folding, trafficking, and degradation of proteins, with specific proteins and mechanisms orchestrating these processes. The endoplasmic reticulum (ER) stands as a primary site for protein folding and quality control. Within its lumen, chaperone proteins such as BiP/GRP78 and calnexin/calreticulin assist in guiding nascent polypeptides into their native conformations [3]. The ER also houses the unfolded protein response (UPR), a signaling pathway activated in response to ER stress, which coordinates adaptive measures to restore proteostasis. In the cytosol, molecular chaperones such as Hsp70 and Hsp90 safeguard protein folding, preventing misfolding and aggregation [4]. The ubiquitin-proteasome system (UPS) plays a vital role in protein degradation, tagging misfolded or damaged proteins with ubiquitin for proteasomal degradation. Mitochondria harbor molecular chaperones like Hsp60 and Hsp70, which facilitate the folding of mitochondrial proteins [5].
Understanding the specific proteins and mechanisms operating within each cellular compartment is essential for deciphering the molecular underpinnings of proteostasis and its dysregulation in disease. By elucidating these intricate pathways, researchers can identify novel therapeutic targets and develop interventions aimed at restoring protein homeostasis in various pathological conditions. Thus, the ability to probe the performance of the proteostasis machinery through detecting aggregation with organelle-level resolution is essential.
Existing tools for studying protein aggregation offer valuable insights, but they come with limitations. While immunoblots can detect total protein levels, they lack spatial information, preventing precise localization of aggregates within the cell beyond crude fractions. Electron microscopy provides high resolution but requires harsh fixation procedures, hindering live-cell studies and potentially altering the delicate ultrastructure of subcellular organelles. Fluorescent microscopy offers the advantage of live-cell imaging but often struggles to distinguish between misfolded aggregates and native protein structures, particularly within crowded confines of organelles such as the ER.
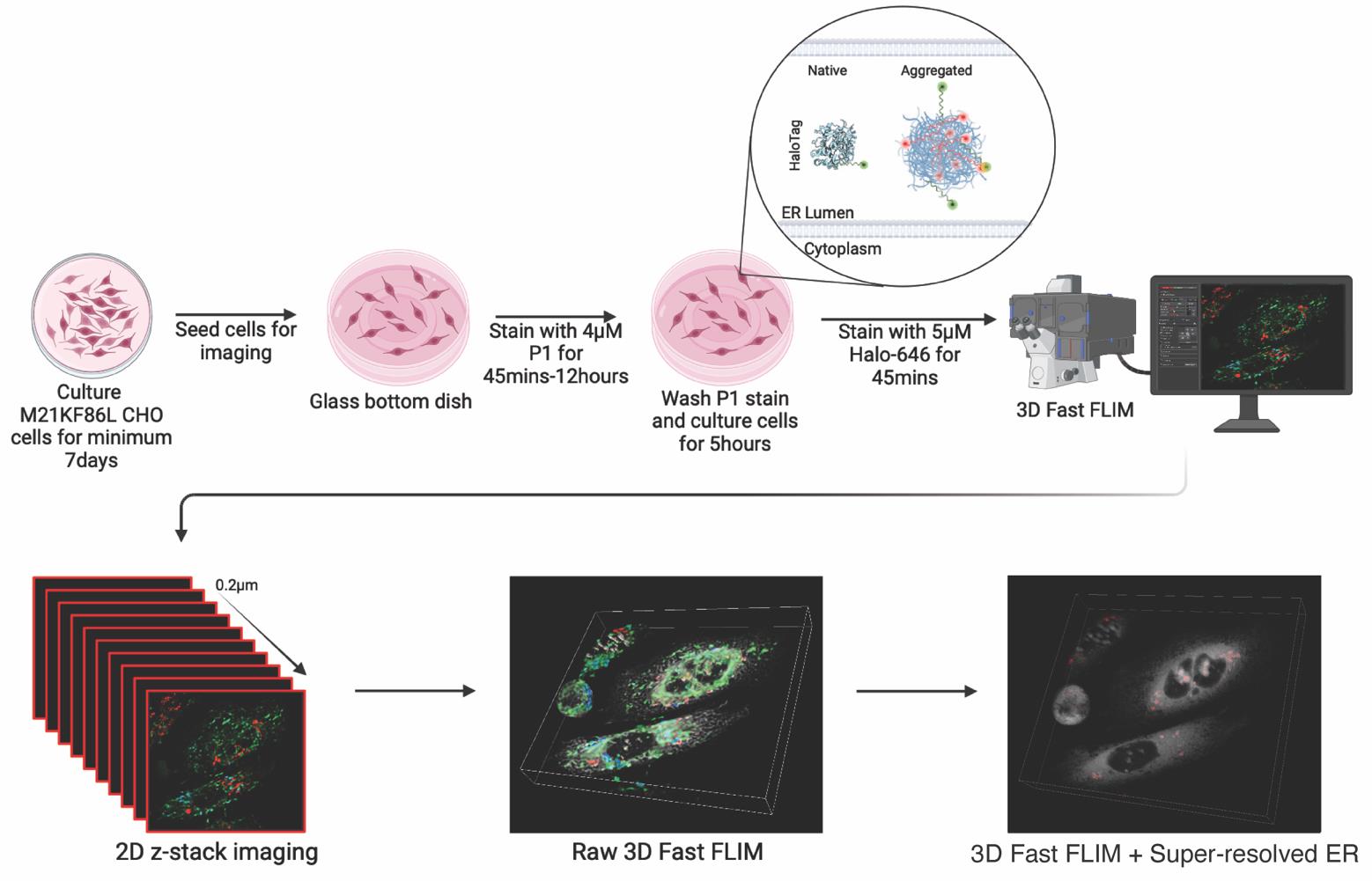
Our protocol addresses these limitations by introducing a subcellular resolution system combining confocal super-resolution microscopy with fast fluorescence lifetime imaging microscopy (FLIM) [6] by taking advantage of a metastable probe labeled with a solvatochromic fluorophore [7] that changes its lifetime depending on aggregation state [8]. Solvatochromic dyes change fluorescent properties based on their microenvironment, namely by polarity. P1 solvatochromic fluorophore is a modified BODIPY with a chloro-alkane chain ligand designed to covalently bind to Halotag, a modified bacterial haloalkane dehalogenase. HT-aggrER is Halotag further modified by the addition of signal peptide and KDEL motif for ER localization and point mutations that make the otherwise metastable protein more aggregate-prone [7]. When P1 binds to and labels HT-aggrER in living cells, its fluorescence lifetime is influenced by protein aggregation status, with a longer lifetime observed in aggregated proteins, which can be promoted by heat shock [8]. Monomeric protein can also be visualized by P1 labeling, but with a shorter lifetime, allowing for optical separation of protein species and thus real-time monitoring of protein aggregation status. HT-aggrER can also be labeled by fluorescent ligands such as TMR and Janelia Fluor 646, which label non-aggregated HT-aggr more brightly than P1 but are quenched and not fluorescent inside hydrophobic aggregates. This provides another degree of flexibility in the labeling of aggregated and soluble protein species. Resolving protein aggregates with this level of detail in live cells is affordable through the critical improvement in speed of time-correlated single-photon counting (TCSPC) fast FLIM on the Leica SP8 FALCON [9,10], which detects photons at ~80 mega counts per second and plots average arrival time for each pixel in near-real time without exponential fitting [11]. LIGHTNING deconvolution applies the Richardson-Lucy algorithm and the underlying principles of point spread function to remove background and out-of-focus signal from a 3D confocal image voxel by voxel, based on an adaptative decision mask. Implementing fast FLIM alongside LIGHTNING deconvolution of correlated confocal images allows for the localization of the FLIM data to sub-diffraction limit resolution counter images. This combination unlocks new avenues for studying protein aggregation state with subcellular resolution, offering several key advantages over existing methods. Beyond providing unparalleled insights into ER protein aggregation, our protocol is applicable for exploring other areas of cellular proteostasis, using the aggregation probe variants targeted to other cellular compartments. Additionally, this system could be used for live-cell drug screening, allowing researchers to rapidly assess the effectiveness of potential therapeutics in preventing or disassembling protein aggregates. The versatile protocol described here thus fills a gap in existing methodologies by offering high-resolution and quantitative insights into the complex protein aggregation process. Its implementation can help to gain a deeper understanding of protein misfolding-related diseases and the development of therapeutic strategies against them.
Materials and reagents
Biological materials
Chinese hamster ovary cells (CHO-K1, ATCC CCL-61) with stable expression of HT-aggrER (HaloTag with M21K F86L mutations)
Nutrient mixture F12 Ham [Sodium bicarbonate (+), L-Glutamine (-)] (Sigma Merck, catalog number: N4888-500ML)
Fetal bovine serum (FBS) (Sigma, catalog number: F9665-500ML)
Penicillin-streptomycin (Pen/strep) (Thermo Scientific, catalog number: 15140122)
L-Glutamine (Thermo Scientific, catalog number: 25030024)
P1 solvatochromic fluorophore (prepared in-house—protocol described in General Notes and Supplementary File 1)
1× Dulbecco’s phosphate-buffered saline (DPBS) (Thermo Fisher Scientific, catalog number: 14190169)
0.05% Trypsin-EDTA (1×) (Thermo Fisher, catalog number: 25200056)
Janelia Fluor 646 HaloTag ligand (Promega, catalog number: GA1120) (see General Notes for further discussion of the use of commercially available HaloTag ligands to label HT-aggr)
Complete nutrient mixture F12 Ham (see Recipes)
P1 staining solution (see Recipes)
Janelia Fluor 646 HaloTag staining solution (see Recipes)
Complete nutrient mixture F12 Ham
Reagent Stock concentration Final concentration Amount Nutrient mixture F12 Ham 100× 90% 500 mL FBS 100× 10% 50 mL Pen/strep 10,000 U/mL 1% 5.5 mL L-Glutamine 200 mM 1% 5.5 mL P1 staining solution
Reagent Final concentration Amount Complete nutrient mixture F12 Ham NA 500 μL P1 (2 mM) 4 μM 1 μL Janelia Fluor 646 HaloTag staining solution
Reagent Final concentration Amount Complete nutrient mixture F12 Ham NA 500 μL Janelia Fluor 646 HaloTag (200 mM) 200 μM 0.5 μL
Laboratory supplies
Coverslip bottomed dishes, IBIDI (IBL Labor GmbH, catalog number: D35C4-20-1-N)
10 cm dish (Falcon, catalog number: 353003)
Centrifuge tube, 15 mL (Appleton Woods Ltd, catalog number: AB031)
Equipment
Leica Stellaris 8 FALCON FLIM Microscope (Leica, Wetzlar, Germany, model: STELLARIS8)
Benchtop centrifuge (Eppendorf, model: 5810 R, product code: 12813252)
Software and datasets
Leica LAS X (v5.2.2, 01.02.2024)
Icy (v2.5.2.0, 08.02.2024)
Procedure
文章信息
稿件历史记录
提交日期: May 10, 2024
接收日期: Aug 8, 2024
在线发布日期: Sep 29, 2024
出版日期: Oct 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Gupta, K., Maddison, D. C., Melo, E. P., Da Costa, A. R. M. and Avezov, E. (2024). Fluorescence Lifetime-Assisted Probing of Protein Aggregation with sub-Organellar Resolution. Bio-protocol 14(19): e5080. DOI: 10.21769/BioProtoc.5080.
分类
生物物理学 > 显微技术
神经科学 > 神经系统疾病 > 神经退行性病变
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link