- EN - English
- CN - 中文
Visualization and Analysis of Neuromuscular Junctions Using Immunofluorescence
使用免疫荧光技术对神经肌肉接头的可视化与分析
发布: 2024年10月05日第14卷第19期 DOI: 10.21769/BioProtoc.5076 浏览次数: 2599
评审: Marion HoggIshita ChandelJosé M. Dias
Abstract
The neuromuscular junction (NMJ) is an interface between motor neurons and skeletal muscle fibers, facilitating the transmission of signals that initiate muscle contraction. Its pivotal role lies in ensuring efficient communication between the nervous system and muscles, allowing for precise and coordinated movements essential for everyday activities and overall motor function. To provide insights into neuromuscular disease and development, understanding the physiology of NMJ is essential. We target acetylcholine receptors (AChR) by immunofluorescence assay (IFA) with α-bungarotoxin (BTX; snake venom neurotoxins binding to AChR) to visualize and quantify the NMJ. Changes in AChR distribution or structure can indicate alterations in receptor density, which may be associated with neuromuscular disorders or conditions that affect synaptic transmission. This protocol provides the methodology for isolating and longitudinally sectioning gastrocnemius muscle for AChR-targeted IFA for confocal microscopy and performing quantitative analysis of NMJs.
Key features
• Visualizes and quantifies NMJs using α-bungarotoxin.
• Utilizes high-resolution confocal microscopy for detailed imaging.
Keywords: Neuromuscular junction (神经肌肉接头)Graphical overview
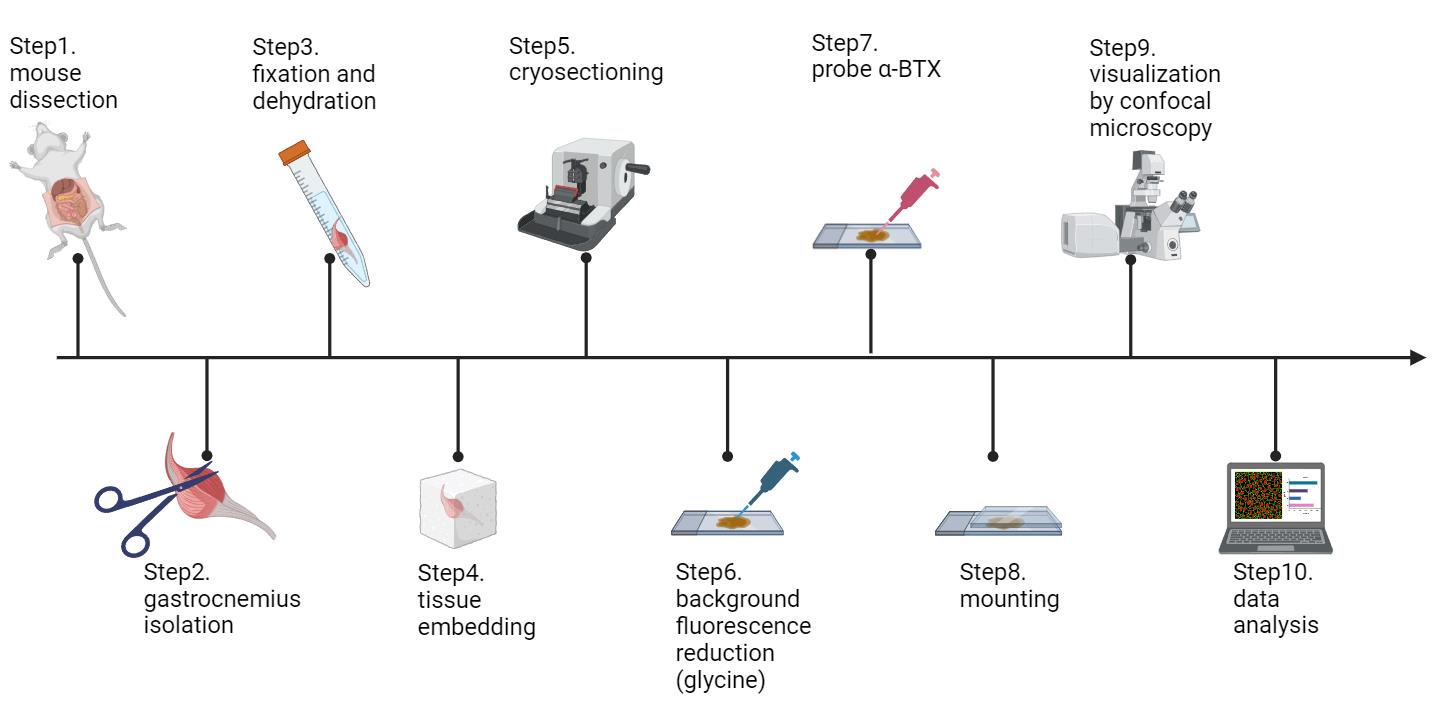
Schematic workflow of this protocol (figure created in BioRender.com)
Background
The neuromuscular junction (NMJ) serves as a chemical synapse between the axon terminal of a motor neuron and the postsynaptic region on a muscle fiber [1]. At the NMJ, the motor neuron's axon terminal releases acetylcholine (ACh) into the synaptic cleft. This neurotransmitter then binds to nicotinic acetylcholine receptors (AChR) on the muscle fiber membrane, causing an influx of sodium ions and subsequent depolarization of the muscle cell membrane. This depolarization initiates an action potential that travels along the muscle fiber, ultimately leading to muscle contraction. The NMJ is vital for voluntary muscle movement and is precisely regulated to ensure accurate motor control [2].
Structural instability of NMJ can cause several neuromuscular diseases and may occur in the aged population to cause loss of muscle strength and mass [3]. For instance, we have recently found that the nuclear receptor interaction protein (NRIP) is an AChR-interacting protein, functioning as a scaffold to stabilize the AChR complex and playing a physiological role in the neuromuscular system [1]. Without NRIP, the efficacy of synaptic transmission and AChR clustering may decline, leading to the loss of nerve supply to NMJs (denervation) and impaired motor function, as observed in NRIP knockout mice (Figure 3C and D in Chen et al. [4]). Additionally, Lrp4 regulates the AChR clustering to guarantee proper NMJ assembly [5]. Conditional knockout of muscle Lrp4 causes a significant decline in AChR cluster size and number [6]. Besides muscle disorder, nerve injury also causes a decrease in AChR cluster and density. Researchers have used a sciatic nerve crush injury model in mice to simulate nerve damage and observed significant fragmentation of the NMJ and a notable decrease in the AChR cluster area [7].
NMJ analysis is a crucial tool in neuromuscular disease research. By combining high-resolution imaging with molecular studies, researchers gain a comprehensive understanding of NMJ physiology, which aids in developing effective treatments for amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), and other neuromuscular diseases. Key visualization techniques include immunofluorescence assays (IFA) with confocal microscopy, electron microscopy, and super-resolution microscopy. IFA with confocal microscopy is preferred for its ability to produce high-resolution 3D images, enabling detailed examination of NMJ structures. α-bungarotoxin, a snake venom toxin, is commonly used to bind acetylcholine receptors for precise visualization. Despite its origin, α-bungarotoxin is safe in labs due to non-toxic concentrations. Confocal microscopy enhances IFA by reducing background noise and increasing image clarity, surpassing traditional fluorescence microscopy. While electron microscopy offers ultra-high resolution, its extensive sample preparation makes it less practical. Super-resolution microscopy provides nanometer-scale visualization but is limited by the complexity and specialized equipment needed. Overall, IFA with confocal microscopy balances high-resolution imaging with ease of use and specificity, making it indispensable in neurobiological research and treatment development for neuromuscular diseases.
Materials and reagents
High-profile microtome blades (Leica, catalog number: 14035838383)
Glass insert 70 mm (Leica, catalog number: 14047742497)
Micro slides (Muto Pure Chemicals, catalog number: GA13-7626R)
O.C.T (VWR, catalog number: 95057838)
Aluminum foil (Kirkland, catalog number: RK611)
10× phosphate-buffered saline (PBS) (Omics Bio, catalog number: IB3012)
Sucrose (Sigma-Aldrich, catalog number: S0389-500G)
Glycine, powder (Omics Bio, catalog number: BT5031)
Paraformaldehyde powder (Sigma-Aldrich, catalog number: P6148-500G)
Alexa-594-conjugated α-bungarotoxin (Life Technologies, catalog number: B13423)
DAPI fluoromount-G (SouthernBiotech, catalog number: 0100-20)
Microscope cover glass (Shinetech Inc, catalog number: GA11-2450)
1.5 mL clear microcentrifuge tubes (Corning, catalog number: MCT-150-C)
2,2,2-tribromoethanol (avertin) (Sigma-Aldrich, catalog number: T48402-5G)
Horse serum (Gibco, catalog number: 16050-114)
BSA albumin fraction V (BioFroxx, catalog number: 4240GR100)
Anti-synaptophysin primary antibody (Abcam, catalog number: ab32127)
Anti-neurofilament primary antibody (Abcam, catalog number: ab8138)
488-conjugated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch, catalog number: AB_2340620)
Solutions
1× PBS (see Recipes)
4% PFA (see Recipes)
2.5% avertin (see Recipes)
0.1 M glycine solution (see Recipes)
30% sucrose (see Recipes)
Blocking buffer (see Recipes)
Recipes
1× PBS
Reagent Final concentration Amount 10× PBS n/a 200 mL Deionized H2O n/a 1800 mL Total n/a 2000 mL Note: The 1× PBS solution should be prepared fresh and used within seven days.
4% PFA
Reagent Final concentration Amount Paraformaldehyde powder 4% 4 g 1× PBS n/a 100 mL Total n/a 100 mL Note: To prepare 4% PFA solution, add 4 g of paraformaldehyde powder to 90 mL of 1× PBS, stir, and heat to 60–65 °C until dissolved. Add a few drops of 5 N NaOH to clear the solution (5 N NaOH is used to facilitate the dissolution of paraformaldehyde powder), cool to room temperature, add 1× PBS to 100 mL, and adjust the pH to 7.4. The solution can be prepared in advance and stored in a -20 °C freezer for long-term storage. Thaw the solution 30 min before use to ensure it is fully liquefied.
2.5% avertin
Reagent Final concentration Amount 2,2,2-tribromoethanol 2.5% 250 μL 1× PBS n/a 9.75 mL Total n/a 10 mL Note: The 2.5% avertin solution can be prepared in advance and stored in a 4 °C refrigerator for up to one month.
0.1 M Glycine solution
Reagent Final concentration Amount Glycine powder 0.1 M 0.75 g 1× PBS n/a 10 mL Total n/a 10 mL Note: To prepare a 0.1 M glycine solution, add 0.75 g of glycine to 10 mL of 1× PBS in a 15 mL conical centrifuge tube and shake until the solution is clear. There is no need to adjust the pH of the solution. The solution can be prepared in advance and stored in a 4 °C refrigerator for up to two months.
30% sucrose
Reagent Final concentration Amount Sucrose 30% 15 g 1× PBS n/a 50 mL Total n/a 50 mL Note: To prepare a 30% sucrose solution, add 15 g of sucrose to 50 mL of 1× PBS and shake until the solution is clear. The solution can be prepared in advance and stored in a 4 °C refrigerator for up to one month.
Blocking buffer
Reagent Final concentration Amount BSA albumin fraction V 2% 0.2 g 1× PBS n/a 9.5 mL Horse serum 5% 500 μL Total n/a 10 mL Note: To prepare the blocking buffer, add 500 μL of horse serum to 9.5 mL of 1× PBS in a 15 mL conical centrifuge tube, shake, and then add 0.2 g of BSA albumin fraction V into the solution. Shake until the solution is clear.
Equipment
P2, P200 and P1000 micropipettes
Laser scanning confocal microscope (Zeiss, model: LSM880)
Staining jar (Shineteh Instruments, catalog number: GB11-15)
Slide racks (Shineteh Instruments, catalog number: GB11-15S)
Liquid nitrogen insulation barrels (Shineteh Instruments, catalog number: IE1-D6000)
Cryostat (Leica, catalog number: CM3050)
4 °C, -20 °C freezers [Sampo, catalog number: SR-C61G(K3)]
-80 °C freezers (Panasonic, catalog number: MDF-U74V)
Laminar flow cabinet (CHUAN, catalog number: TBH-520M)
Orbital shaker (TKS, catalog number: OS-701)
Disposable syringe with needle (Terumo, catalog number: MDSS01S2613)
Disposable syringe (Terumo, catalog number: MDSS20ES)
Iris scissors (Shineteh Instruments, catalog number: ST-S009)
Fine curve point tweezers (Shineteh Instruments, catalog number: ST-NO7)
Super fine point tweezers (Shineteh Instruments, catalog number: ST-NO3)
Iris forceps (Shineteh Instruments, catalog number: ST-I210)
Lab mat (Dogger, catalog number: D4EG-031055)
FalconTM 15 mL conical centrifuge tubes (Thermo Fisher, catalog number: 14-959-53A)
Immuno-pathology staining kit (PEOPLE LIFE TEK CO., LTD., catalog number: H198099910)
Software and datasets
ImageJ image processing and analysis software (https://imagej.net/ij/)
ZEN microscopy software (https://www.zeiss.com/microscopy/en/products/software/zeiss-zen.html)
GraphPad Prism 8.0.2
Procedure
文章信息
稿件历史记录
提交日期: Apr 30, 2024
接收日期: Aug 11, 2024
在线发布日期: Sep 12, 2024
出版日期: Oct 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Hsieh, Y. T. and Chen, S. L. (2024). Visualization and Analysis of Neuromuscular Junctions Using Immunofluorescence. Bio-protocol 14(19): e5076. DOI: 10.21769/BioProtoc.5076.
分类
神经科学 > 基础技术 > 组织解剖
神经科学 > 神经解剖学和神经环路 > 荧光成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link