- EN - English
- CN - 中文
A Full Good Manufacturing Practice–Compliant Protocol for Corneal Stromal Stem Cell Cultivation
符合GMP标准的角膜基质干细胞培养全流程
发布: 2024年09月20日第14卷第18期 DOI: 10.21769/BioProtoc.5074 浏览次数: 2148
评审: Alessandro DidonnaShun Yu Jasemine YangGabriel Campolina-Silva
Abstract
Corneal scarring, a significant cause of global blindness, results from various insults, including trauma, infections, and genetic disorders. The conventional treatment to replace scarred corneal tissues includes partial or full-thickness corneal transplantation using healthy donor corneas. However, only 1 in 70 individuals with treatable corneal scarring can undergo surgery, due to the limited supply of transplantable donor tissue. Our research focuses on cell-based strategies, specifically ex vivo–expanded corneal stromal stem cells (CSSCs), to address corneal scarring. Preclinical studies have demonstrated the efficacy of CSSC treatment in reducing corneal inflammation and fibrosis, inhibiting scar formation, and regenerating native stromal tissue. Mechanisms include CSSC differentiation into stromal keratocytes and the expression of regenerative cytokines. Here, we present a good manufacturing practice (GMP)-compliant protocol to isolate and expand human CSSCs. This method paves the way to produce clinical-grade CSSCs for transplantation and clinical trials.
Key features
• This protocol utilizes surgical skills to dissect human corneal tissues for CSSC isolation.
• The yield and features of CSSCs rely on donor tissue quality (freshness) and have donor-to-donor variability.
• Up to 0.5 billion CSSCs can be generated from a single cornea specimen, and cells at passage 3 are suitable for treatment uses.
Keywords: Corneal opacities (角膜混浊)Graphical overview
Background
Damage to the cornea, caused by physical trauma, chemical burns, infections such as microbial or viral keratitis, or genetic disorders like corneal dystrophies and keratoconus, initiates a complex cascade of inflammatory and fibrotic reactions [1,2]. These events lead to tissue damage and remodeling, which involves the generation of stromal fibroblasts and myofibroblasts. These cells produce a repair-type extracellular matrix (ECM) to heal the wound. However, excessive deposition of ECM, including type-III collagen and fibronectin extradomain A (EDA-Fn), leads to the formation of haze and opacities, eventually resulting in corneal scarring that can compromise the corneal clarity and integrity, causing visual impairment [3]. While superficial scarring can often heal within months, deep or dense scars may develop and cause permanent vision loss.
Corneal scarring is the fourth leading cause of global blindness, affecting approximately 2 million people [4]. Each year, over 350,000 children are affected by this condition [5,6]. This is a major concern, as it disproportionately affects younger individuals and low-income rural communities due to factors such as communicable diseases, higher risk of injury, and limited access to treatment options [7]. Various options are available to treat scarred corneas, including pharmacological treatments such as steroids, immune modulation, and grafting with donor corneal materials [8]. Pharmacological therapies offer moderate correction for corneal haze and mild opacities. Corneal transplantation, such as penetrating and lamellar keratoplasty, is the standard for moderate-to-severe scarring and opacities occurring along the visual axis. However, the limited global supply of transplantable donor corneas, immune response (depending on the histocompatibility context of the host that could affect the long-term graft survival), risk of graft rejection, and potential surgical complications restrict the widespread use of keratoplasty [7,9].
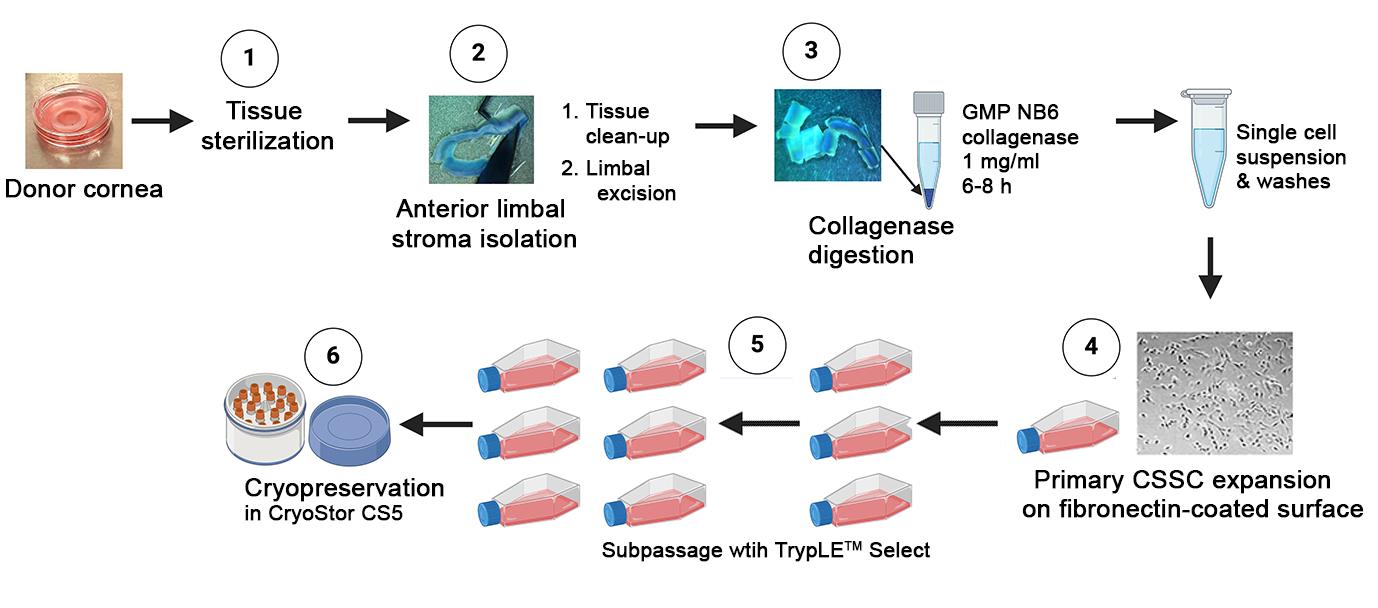
Our research team investigated the use of human corneal stromal stem cells (CSSCs) to reduce corneal inflammation and fibrosis; the treatment prevented corneal scarring and restored corneal clarity in pre-clinical models of stromal injury created by mechanical debridement, alkali burns, or liquid nitrogen [10–17]. Our team follows a specific set of protocols to isolate primary donor CSSCs through microdissection of the anterior limbal stroma and then culture them using a stem cell growth medium called JM-H [10,18,19]. These CSSCs can be propagated up to 50–70 doublings before undergoing senescence and changes in cell features [20]. They express stem cell markers like ATP-binding cassette transporter superfamily G member 2 [ABCG2], nestin, Pax6, and Bmi-1, undergo stromal keratocyte differentiation, express anti-inflammatory tumor necrosis factor-stimulating gene 6 [TSG-6] and transforming growth factor b3 [TGFb3], and anti-fibrosis microRNAs (hsa-miR-29a and 381) [10,12,14,17,18].
In order to apply these cells in clinical settings, it is necessary to generate them using a good manufacturing practice (GMP)-compliant protocol. Regulatory approval for cell therapy is challenging, with only a small number gaining approval in comparison to pharmaceutical drugs [21]. Quality, safety, and effectiveness are extremely important for clinical trials, guided by good clinical practice principles [22,23]. To ensure regulatory compliance and consistent results, a quality management system that encompasses quality assessment and quality control is crucial for cell therapy [23]. We have developed GMP-compliant protocols for clinical-grade CSSC production, which laid the groundwork for human testing in clinical trials.
Materials and reagents
Biological materials
Donor corneas
GMP Reagents
Dulbecco’s modified Eagle medium (DMEM/F12) (Thermo Fisher Scientific, Gibco, catalog number: 12634-010)
Phosphate buffered saline (PBS) (Thermo Fisher Scientific, Gibco, catalog number: 10010-023)
DMEM (1 g/L D-glucose, with GlutaMAX) (Thermo Fisher Scientific, Gibco, catalog number: 10567-014)
Ham’s F12 (Thermo Fisher Scientific, Gibco, catalog number: 31765-035)
BioWhittaker antibiotic-antimycotic (100×) (Lonza, catalog number: 17-745E)
Insulin-transferrin-selenium (100×) (Thermo Fisher Scientific, Gibco, catalog number: 41400-045)
Flexbumin (25%) (BioSupply, catalog number: 00944-0493-01)
2-Phospho-L-Ascorbate (Tocris Biosci, catalog number: 5778)
Recombinant human epidermal growth factor (EGF) (Thermo Fisher Scientific, Gibco, catalog number: PHG6045)
Recombinant human platelet-derived growth factor-BB (PDGF-BB) (R&D, catalog number: 220-GMP-050)
Dexamethasone (Dex) (USP micronized) (Spectrum, catalog number: DE121)
Human serum (Innovative Res, catalog number: ISER-36670)
Collagenase (Collagenase NB6) powder (Nordmark, catalog number: N0002779)
Fibronectin coating substrate (MedChemExpress, catalog number: HY-P70593G)
TrypLE Select CTS (Thermo Fisher Scientific, Gibco, catalog number: A12859-01)
CryoStor CS10 (serum-free DMSO 10%) (BioLife Solutions, catalog number: 210373)
Trypan blue (0.4%) (Invitrogen, catalog number: T10282)
Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: H9892)
Solutions
Recombinant human EGF (see Recipes)
Recombinant human PDGF (see Recipes)
Dexamethasone (Dex) solution (see Recipes)
Collagenase NB6 solution (see Recipes)
GMP fibronectin (see Recipes)
CryoStor CS5 (see Recipes)
GMP stem cell culture medium (see Recipes)
Recipes
Recombinant human EGF
Store the lyophilized EGF at -20 °C under desiccation until reconstitution. Prior to opening, briefly centrifuge the vial of lyophilized EGF at 400× g for 10–15 s to bring the contents to the bottom. Reconstitute EGF powders (500 mg) with sterile distilled water (1 mL volume) to a concentration of 0.5 mg/mL. Further dilute to 10 mg/mL with sterile DMEM/F12 added with 0.1% Flexbumin (carrier protein), aliquot at 0.5 mL, and store at -80 °C until use or at 2–8 °C for one month.
Reagent Final concentration Quantity Recombinant human EGF 10 mg/mL 100 mL from 0.5 mg/mL stock DMEM/F12 1× 5 mL Flexbumin 0.1% (v/v) 20 mL from 25% stock Recombinant human PDGF
Store the lyophilized PDGF-BB at -20 °C under desiccation until reconstitution. Prior to opening, briefly centrifuge the vial of lyophilized PDGF-BB at 400× g for 10–15 s to bring the contents to the bottom. Reconstitute PDGF-BB powders (50 mg) with sterile hydrochloric acid (HCl, 4 mM) with 0.1% Flexbumin (carrier protein) to a concentration of 10 mg/mL, aliquot at 0.5 mL, and store at -80 °C until use or at 2–8 °C for one month.
Reagent Final concentration Quantity Recombinant human PDGF-BB 10 mg/mL One vial of 50 mg HCl 4 mM 5 mL Flexbumin 0.1% (v/v) 20 mL from 25% stock Dexamethasone (Dex) solution
Store the lyophilized Dex at -20 °C under desiccation until reconstitution. Prior to chemical preparation, wear PPE and a suitable eye protection device as Dex has a risk of causing eye damage/irritation. Thaw Dex at room temperature for 10–20 min. Weigh approximately 50 mg of powder and calculate the amount of distilled water to make a stock concentration of 10 mM with reference to the molecular weight 392.47. Further dilute to 10 mM with sterile DMEM/F12, aliquot at 100 mL volume, and store at -80 °C until use or at 2–8 °C for one month.
Reagent Final concentration Quantity Dexamethasone 10 mM 5 mL from 10 mM stock DMEM/F12 1× 5 mL Collagenase NB6 solution
Store the lyophilized collagenase powder at 2–8 °C under desiccated conditions. Dissolve 100 mg of powder with 10 mL of sterile PBS (cGMP) to make a stock concentration of 10 mg/mL. Aliquot the solution to 0.15 mL in sterile 2 mL Eppendorf tubes and store at -20 °C. Prior to tissue digestion, thaw the collagenase aliquot at room temperature for 10–15 min and add 1.35 mL of DMEM/F12 with 0.1% Flexbumin and 1× antibiotics/antimycotic to make a working concentration of 1 mg/mL.
Reagent Final concentration Quantity Collagenase NB6 powder 1 mg/mL 0.15 mL of 10 mg/mL stock DMEM/F12 1× 1.35 mL Flexbumin 0.1% (v/v) 6 mL Antibiotics/antimycotic 1× 15 mL from 100× stock GMP fibronectin
Store lyophilized fibronectin at -20 °C under desiccation for a maximum of 2 years. Thaw the powder at room temperature for 10–20 min. Prior to opening, briefly centrifuge the vial at 400× g for 10–15 s to bring the contents to the bottom. Add 1 mL of injection water to 500 mg of fibronectin powder to make a stock concentration of 500 mg/mL, aliquot to 50 mL volume, and store at -80 °C for up to a year or at 2–8 °C for a week.
Fibronectin coating: Dilute GMP fibronectin at 500 mg/mL stock with sterile PBS to 10 mg/mL working concentration. Apply 1 mL per TCF25 (tissue culture flask, 25 cm2) and coat at room temperature for 15–30 min. Remove the solution, and the surface is ready for cell seeding.
Reagent Final concentration Quantity Fibronectin 10 mg/mL 20 mL of 500 mg/mL stock PBS 1× 1 mL CryoStor CS5
Store CryoStor CS10 at 2–8 °C. Prior to use for CSSC cryopreservation, dilute CS10 with GMP culture medium (1:1 v/v) to make a working CS5 solution with 5% DMSO and keep at room temperature for immediate cell storage or at 2–8 °C for one week. The step of cell freezing requires the use of a controlled temperature apparatus (e.g., Mr. FrostyTM freezing container with isopropanol).
Reagent Final concentration Quantity GMP culture medium 0.5× 0.5 mL CryoStor CS10 0.5× 0.5 mL GMP stem cell culture medium (GMP SCCM)
Thaw the frozen reagents (including recombinant human EGF, recombinant human PDGF-BB, and Dex) at room temperature for approximately 10–15 min or at 2–8 °C overnight. Mix the thawed solutions by gently inverting the Eppendorf vial a few times, followed by a brief spinning with a benchtop micro-centrifuge.
To prepare SCCM with 500 mL volume, mix 300 mL of DMEM (1,000 mg/L D-glucose) and 200 mL of Ham’s F12. Add reagents with the required volume, filter-sterilize, and bring the culture medium to room temperature before use.
Reagents Final concentration Volume DMEM (1 g/L D-glucose, with GlutaMAX) 60% (v/v) 290 mL Ham’s F12 40% (v/v) 190 mL BioWhittaker antibiotic-antimycotic (100×) 1× 5 mL Insulin-transferrin-selenium (100×) 0.5× 2.5 mL 2-Phospho-L-Ascorbate (100 mM) 0.5 mM 2.5 mL Recombinant human EGF (10 mg/mL) 10 ng/mL 0.5 mL Recombinant human PDGF-BB (10 mg/mL) 10 ng/mL 0.5 mL Dexamethasone, USP micronized (10 mM) 10 nM 0.5 mL Flexbumin (25%) 0.1% (v/v) 2 mL Human serum (100%) 2% (v/v) 10 mL Notes:
All reagents used in this GMP culture formulation adhere to the GMP standards. During the development of the GMP-compliant formulation, we tested different GMP substitutes for the research-grade chemicals/reagents that are used in the lab-based culture medium [19]. This was done to ensure that the formulation meets the GMP standard. The corresponding reagents were comprehensively evaluated against the original research-grade components through rigorous cellular and molecular assays using at least three primary donor CSSC batches. After evaluating multiple GMP-grade reagents, we meticulously developed an optimized formulation that closely mirrors the research-grade culture medium.
It is worth noting that human serum can be lipid- and protein-rich, depending on the donors. This can cause the culture medium to appear cloudy with the presence of insolubilities, although it is sterile. For this reason, we suggest that the final medium should be filter-sterilized before use.
Laboratory supplies
Cell strainer (40 and 70 μm pore size) (Fisher Scientific, catalog numbers: 22362537 and 22362548)
Cryovials (1.8 mL volume) (Fisher Scientific, catalog number: 1050026)
Tissue culture flask (TCF) 25 cm2 (Corning, catalog number: 430168), 75 cm2 (Genesse Scientific, GenClone, catalog number: 25-209); tissue culture dish (TCD) 30 mm (Falcon, catalog number: 353001), 60 mm (Falcon, catalog number: 353002), 100 mm (diameter) (Genesse Scientific, GenClone, catalog number: 25-202); tissue culture plate (TCP) 6-well (Fisher Scientific, catalog number: EB012927)
Sterile syringe (10 mL volume) (BD, catalog number: 305482)
Sterile centrifuge tubes (15 mL and 50 mL volume) (VWR, catalog numbers: 525-1069 and 525-1014)
Sterile microcentrifuge tubes (1.5 mL and 2 mL volume) (Fisher Scientific, catalog numbers: 05-408-129 and 02-681-321)
Sterile syringe filters (0.22 mm pore size) (Nalgene, catalog number: 726-2520)
Equipment
Biological safety cabinet (Class II type A2/B2) (Baker, model: SteriGARD III Advance)
Phase-contrast microscope with 4×, 10×, and 20× objectives (Invitrogen, model: EVOS XL Core)
Stereomicroscope for dissection (0.8 to 16×) (AMScope, model: SM-1 LED 1445)
Humidified CO2 incubator (Thermo, model: HERAcell 150i)
Temperature-controlled benchtop centrifuge (Thermo, catalog number: 75004381)
Automated cell counter (Invitrogen, model: CountessTM)
Colibri toothed forceps
Vannas curved scissors (6 mm blade) for corneal procedures
Scalpel handle #3
Sterile surgical blade #10 (Integra Miltex carbon steel sterile surgical blades, model: 4-110)
Germinator glass bead sterilizer (Fisher Scientific, model: Microbead Sterilizer B1305-FIS)
Rotator (set at 30 rounds per minute, rpm)
Mr. FrostyTM freezing container (with isopropanol) (Nalgene Cryo freezing container, model: 5100-0001)
Liquid nitrogen cell storage (Thermo, model: Thermolyne CY509975)
Digital balance (Denver Inst Co., model: TR2102)
Procedure
文章信息
稿件历史记录
提交日期: Apr 30, 2024
接收日期: Aug 11, 2024
在线发布日期: Sep 6, 2024
出版日期: Sep 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Santra, M., Hsu, Y. S., Khadem, S., Radencic, S., Funderburgh, M. L., Sawant, O. B., Dhaliwal, D. K., Jhanji, V. and Yam, G. H. F. (2024). A Full Good Manufacturing Practice–Compliant Protocol for Corneal Stromal Stem Cell Cultivation. Bio-protocol 14(18): e5074. DOI: 10.21769/BioProtoc.5074.
分类
干细胞 > 成体干细胞 > 间充质干细胞
细胞生物学 > 细胞分离和培养 > 细胞生长
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link