- EN - English
- CN - 中文
Development and Characterization of Primary Brain Cultures from Japanese Quail Embryos
日本鹌鹑胚胎原代脑细胞培养的开发与特性分析
(*contributed equally to this work) 发布: 2024年09月20日第14卷第18期 DOI: 10.21769/BioProtoc.5071 浏览次数: 1611
评审: Sébastien GillotinSilvia Olivera-BravoHong Lian
Abstract
Cell cultures play a crucial role in neuroscience research, facilitating the elucidation of the complexities of cellular physiology and pathology. The relative simplicity in producing cultures and the accessibility to cells that the cultures provide, in contrast to in vivo settings, allow users to manipulate and monitor cells more easily at higher throughputs and lower costs. These are ideal for screening purposes and electrophysiological characterizations. Despite the prevalence of methodologies for producing brain cultures from various animal models, rodents in particular, approaches for culturing neurons (and glia) from birds are less established or completely absent as in the case of the Japanese quail model. Here, we present a unique culturing protocol for brain cells (e.g., neurons at different maturation levels, such as progenitor cells, excitatory and inhibitory neurons, microglia, and endothelial cells) from entire forebrains of Japanese quail embryos for high-throughput screening of viral vectors in vitro and other various purposes. Following dissection and digestion methods uniquely suited for avian brains, we tailored the growth media and culturing surface to allow the survival of quail brain cultures for more than three weeks in vitro.
Key features
• We introduce a detailed protocol for producing primary brain cultures from quail embryos' forebrains for up to 30 days.
• We show that the cultures support in vitro viral transfections effectively.
• We demonstrate the use of the cultures for rapid (days) screening for suitable viruses for quail brain cells, electrophysiological characterizations, and single mRNA sequencing.
Keywords: Primary cell culture (原代细胞培养)Graphical overview
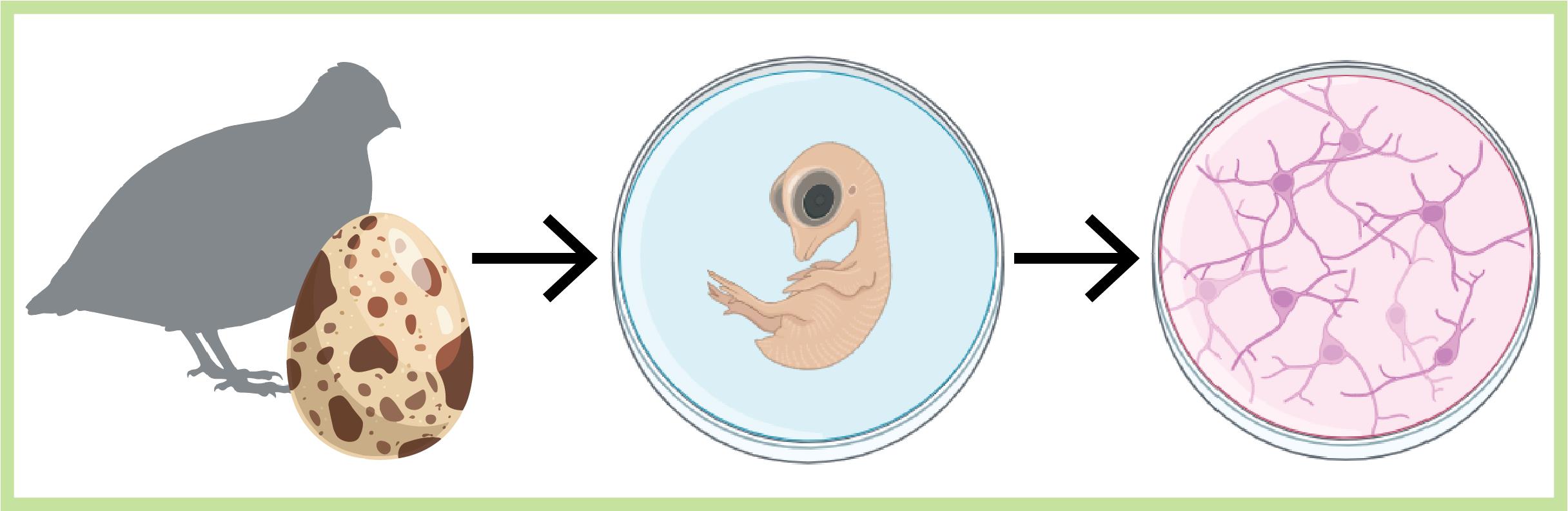
Background
Bird species offer intriguing prospects for neuroscience research due to their sophisticated cognitive abilities and specialized behaviors [1–6]. Despite differences in neuroarchitecture and neuronal densities in avian brains when compared with mammals [4,7], birds demonstrate remarkable and distinctive behavioral performances, including long-distance navigation [8], imprinting, homing, food-caching, and song-learning [9,10], to name a few. Consequently, these provide unique opportunities for comparative studies aiming to elucidate the cellular mechanisms underlying these capabilities [6]. Indeed, these realizations are reflected by the growing interest in avian neuroscience [1,3,9,11–18]. Among the different avian species explored, our focus centers on the domestic Japanese quail (Coturnix japonica). The quail offers several advantages over other birds due to its small size, rapid sexual maturation, and frequent egg-laying; advantages that make this species highly suitable for routine experimentations [17]. Importantly, the ground-dwelling nature of quails makes them ideal for studying birds’ spatial navigation by reducing dimensionalities (from 3D to 2D) [19]. In spite of these advantages and the extensive use of quails in the field of developmental biology [17,20], this species remains relatively underexplored in neuroscience [5,19]. This is partly attributed to the absence of methods and tools to manipulate and examine quail neurons [21], in particular, the lack of efficient viral vectors for neuronal transduction [5,17]. To screen for suitable viral vectors, we choose not to pursue in vivo exploration, as is common practice, due to the considerably large number of animals and time this task necessitates (to overcome animal-to-animal variability, variable injection performances, and other technical limitations). Instead, we envisioned that primary brain cultures would present a more rapid and cost-effective approach for examining multiple viruses in parallel [22].
Primary brain cell cultures constitute a valuable tool in neuroscience [23,24]. Brain cultures faithfully preserve many complex cellular behaviors, electrophysiological properties, and phenotypic traits, as observed in vivo, despite the loss of the precise network architecture and organization in the tissue, as is exclusively found in vivo [23-28]. Nevertheless, the alteration in tissue architecture, which includes the removal of the blood-brain barrier (BBB) and extracellular matrix, proves to be advantageous as it enhances cellular accessibility and reduces time to expression. Indeed, cultures are ideal for high-throughput screening of pharmacology, which should likely translate toward the screening of different viruses [22].
We have initially attempted to produce primary cultures based on standard protocols for culturing cells from the brains of neonatal rodents (such as rats and mice, e.g., [29]) or chicken embryos (e.g., [30,31]). However, these attempts were unsuccessful. Consequently, we decided to optimize the procedure by experimenting with embryos collected from different days in ovo (DIO), modifying the culturing media, and assessing cell growth directly on plastic dishes instead of glass coverslips. After successfully establishing suitable conditions for maintaining viable cultures for several weeks (up to 30 days), we leveraged them for screening several commonly employed viruses in neuroscience research, including adeno-associated viruses (AAV), lentiviruses, and baculovirus. We further harnessed the cultures for the rational engineering of a novel AAV variant, specifically tailored for Japanese quail neurons (denoted AAV1*) [22]. Lastly, we characterized the diverse cellular populations in the cultures through use of electrophysiology, calcium imaging, and single-cell mRNA sequencing [22].
Materials and reagents
Biological materials
Fertilized Japanese quail eggs (Coturnix japonica). The eggs we employ are obtained from our quail colony. Eggs are collected from cages with male and female quails. Nevertheless, there are multiple commercial providers of fertilized quail eggs; however, the number of farms and thereby commercial availability may vary from one country to another.
Reagents
B-27 serum-free supplement (50×) liquid (Gibco, catalog number: 17504-044)
Pen/Strep solution (Sartorius, catalog number: 03-031-1B)
L-glutamine (GlutaMAX) (Gibco, catalog number: 35050-038)
Neurobasal-A medium (Gibco, catalog number: 10888-022)
Papain from papaya latex (Sigma-Aldrich, catalog number: 9001-73-4)
Phosphate buffered saline (PBS) (Sartorius, catalog number: 02-023-5A)
Poly-D-lysine hydrobromide (PDL) (Sigma-Aldrich, catalog number: 27964-99-4)
DNase I set (Geneaid, catalog number: DNS300)
Sylgard 184 silicone elastomer (Sigma-Aldrich, catalog number: 761036-5EA)
DMEM/F-12 (Ham) (Sartorius, catalog number: 01-170-1A)
Solutions
0.2% PDL (see Recipes)
Quail neurobasal medium (QNBM) (see Recipes)
Digestion solution (see Recipes)
Papain stock (see Recipes)
Recipes
Note: Prepare all solutions in a laminar flow cell culture hood.
0.2% PDL (store at -20 °C)
Reagent Final concentration Amount PDL 2 mg/mL 100 mg Double-distilled water (DDW) n/a 50 mL Total n/a 50 mL Quail neurobasal medium (NBM) (store at 4 °C)
Reagent Final concentration Amount B-27 2% 5 mL Pen/Strep 1% 2.5 mL L-glutamine 0.25% 625 µL Neurobasal-A medium n/a Complete to 250 mL Total n/a 250 mL Digestion solution (prepare on the day of extraction)
Reagent Final concentration Amount Papain stock 33 U/mL 1 mL PBS n/a 2 mL DNase I 57 U/mL 85 µL Total n/a 3.085 mL Papain stock
Reagent Final concentration Amount Papain 10 U/mg 100 mg PBS n/a 10 mL Total n/a 10 mL
Laboratory supplies
Spray bottle with 70% ethanol (Bio-Lab Chemicals, catalog number: 000521020500)
Tissue culture dish (60 mm × 15 mm) (Corning, catalog number: 430166)
Tissue culture dish (100 mm × 20 mm) (Corning, catalog number: 430167)
Microtubes, 1.7 mL (Corning, catalog number: MCT-175-C)
Conical bottom tubes, 15 ml (Greiner Bio-One, catalog number: 188261)
Conical bottom tubes, 50 ml (Greiner Bio-One, catalog number: 227270)
Cell strainer 40 μm (LifeGene, catalog number: LG-CSS010040S)
Parafilm (Sigma-Aldrich, catalog number: P7543)
Delicate task Kimwipes (Kimberly-Clark professional, catalog number: 34120)
Minutien Pins, 1 cm, 0.0175 mm tip (Fisher Scientific, catalog number: 2600215)
Equipment
Stereomicroscope system (Zeiss Stemi 2000, Fisher Scientific, catalog number: 10331390)
Microbiological safety cabinet class II (Thermo Scientific, catalog number: 51025757)
Isotemp water bath (PolyScience, catalog number: E12020004)
HERAcell 150i CO2 incubator (Thermo Fisher Scientific, catalog number: 50116047)
Gilson (or equivalent) pipettes and tips (P20/P200/P1000) (Fisher Scientific)
Dumont #5 forceps (Roboz surgical store, catalog number: RS-4905) (Figure 1, #1)
Curved dissecting scissors (Stoelting, catalog number: 52132-11P) (Figure 1, #2)
Plastic Pasteur pipette (blunt tip obtained by trimming pipette ~1 cm from tip) (Sigma-Aldrich, catalog number: 747775 (Figure 1, #3)
Vannas spring scissors (Fine Science Tools, catalog number: 15000-08) (Figure 1, #4)
Iris forceps (Fine Science Tools, catalog number: 11064-07) (Figure 1, #5)
Fine-angled forceps (Stoelting, catalog number: 52102-02P) (Figure 1, #6)
Egg incubator, 37 °C (DMP Engineering, model: INCA 100)
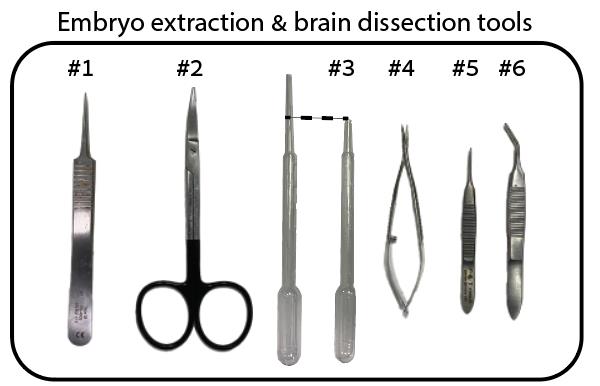
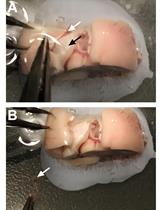
Figure 1. Fine surgical tools required for the removal of eggshell and embryo from egg
Procedure
文章信息
稿件历史记录
提交日期: May 15, 2024
接收日期: Aug 11, 2024
在线发布日期: Aug 21, 2024
出版日期: Sep 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Zoabi, S., Blau, A. and Berlin, S. (2024). Development and Characterization of Primary Brain Cultures from Japanese Quail Embryos. Bio-protocol 14(18): e5071. DOI: 10.21769/BioProtoc.5071.
分类
神经科学 > 细胞机理 > 组织分离与培养
细胞生物学 > 模式生物培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link