- EN - English
- CN - 中文
Mouse Renal Artery Catheterization for Local Delivery of Drugs in Capsulated or Free Forms
用于包封或游离形式药物局部递送的小鼠肾动脉插管
发布: 2024年09月20日第14卷第18期 DOI: 10.21769/BioProtoc.5070 浏览次数: 1456
评审: Olga KopachKomuraiah MyakalaAnonymous reviewer(s)
Abstract
Arterial delivery to the kidney offers significant potential for targeted accumulation and retention of cells, genetic material, and drugs, both in free and encapsulated forms, because the entire dose passes through the vessels feeding this organ during the first circulation of blood. At the same time, a detailed study on the safety and effectiveness of developed therapies in a large number of experimental animals is required. Small laboratory animals, especially mice, are the most sought-after in experimental and preclinical testing due to their cost-effectiveness. Most of the described manipulations in mice involve puncturing the walls of the abdominal aorta or renal artery for direct administration of solutions and suspensions. Such manipulations are temporary and, in some cases, result in long-term occlusion of the aorta. Ultimately, this can lead to disruption of blood flow as well as functional and morphological changes to the kidneys. In addition, few of these protocols describe targeted delivery to the kidney. The presented protocol involves the injection of test substances or suspensions through the renal artery into one of the kidneys. The catheter is implanted into the femoral artery and then advanced into the abdominal aorta and renal artery within the vessels. In this case, the integrity violation of the renal artery or abdominal aorta is absent. Occlusion of the renal artery is necessary only immediately at the time of injection to minimize the entry of the injected substance into the aorta. This protocol is similar to the clinical procedure for delivering a catheter into the renal artery and is designed for real-world operating conditions.
Key features
• The protocol involves implantation of a catheter into the vascular system through a puncture of the femoral artery, similar to the clinical procedure.
• The catheter is moved inside the vessels without puncture or ligation to the aorta or renal artery.
• The protocol involves only a short-term block of the blood supply to the target kidney (the time required for direct administration of the drug).
• Suitable for chronic experiments on mice, since the catheter is removed from the vascular system immediately after drug administration.
Keywords: Femoral artery catheterization (股动脉插管)Graphical overview
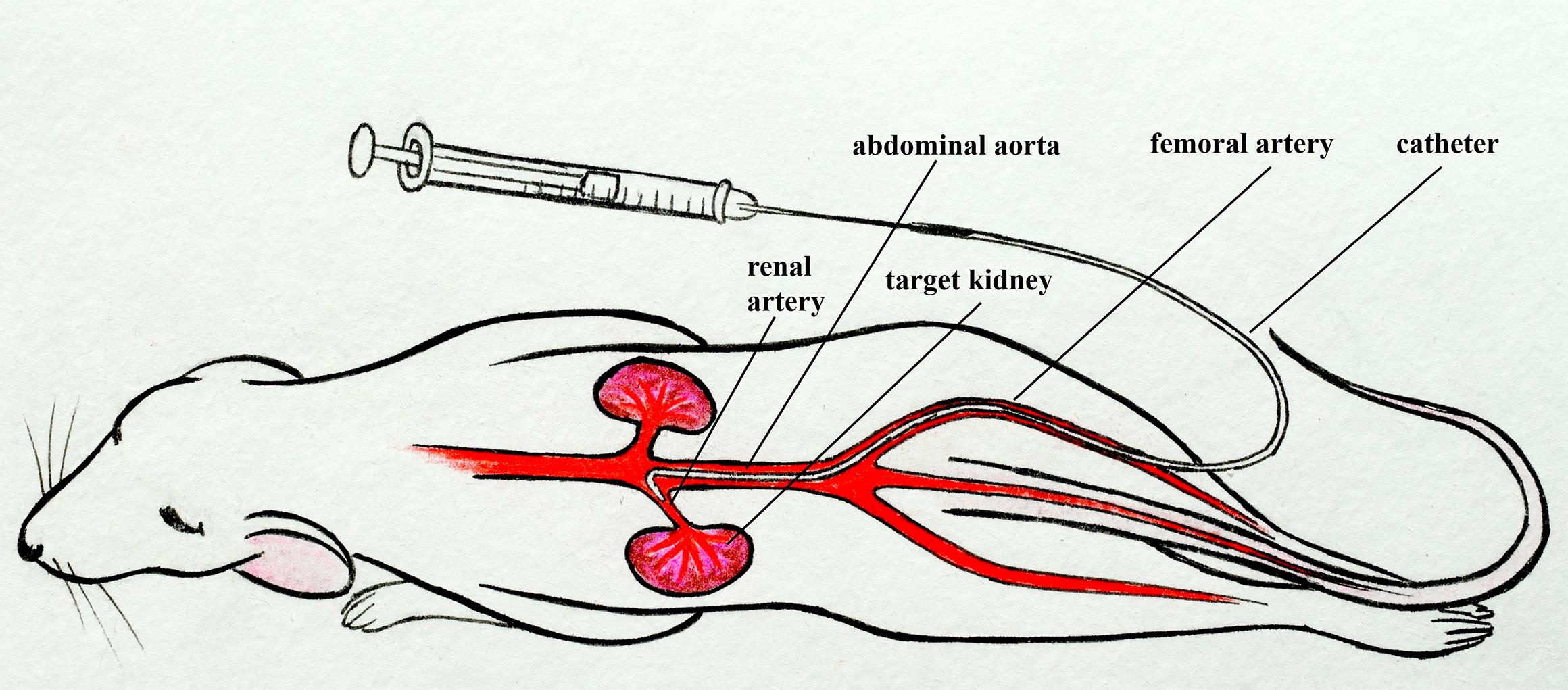
Mouse renal artery catheterization through the femoral artery
Background
Arterial delivery of drugs to the kidneys in free and encapsulated forms has enormous potential to increase local drug concentrations and reduce the side effects of therapy on other organs. The choice of therapy parameters (active substance, pharmaceutical form, dosage regimen, volume, etc.), as well as understanding drug accumulation and retention in the kidneys during arterial delivery, requires a wide range of complex studies on animals. In this regard, small laboratory animals, especially mice, are the most attractive and economical.
The success of the study directly depends on the correctness of the arterial delivery procedure, which is especially difficult for mice due to their small size. Incorrectly performed injections in the renal artery could disrupt the kidney blood supply and lead to tissue damage. The vast majority of drug delivery protocols through the renal artery are based on ligation and direct injection into the abdominal aorta [1]. Chen et al. reported that direct injection of the rAAV2-GFP vector into the rat abdominal aorta using 45 min ligation of renal vein and abdominal aorta led not only to successful transfection of tubular epithelial cells but also to focal areas of chronic interstitial infiltration with mononuclear cells, tubular atrophy, and scar formation [2]. Authors suggested that these changes are most likely the result of ischemic injury inherent in the procedure and not a consequence of recombinant adeno-associated viral vector administration or an adverse immune response to it or the transgene. A similar protocol for mice with short-term occlusion (several minutes) and puncture of the abdominal aorta was presented by Dahlqvist et al. [3]. Zaw Thin et al. developed a percutaneous minimally invasive ultrasound-guided renal artery injection to enhance cell delivery to mice kidneys [4]. This procedure appears to be less invasive (only puncture of muscles and skin with a syringe) than the others but requires a special ultrasound imaging system. In addition, in this protocol, the renal artery wall is punctured with a 29-gauge needle (0.33 mm diameter). Note that the diameter of the mouse renal artery is in the range of 0.3–0.4 mm [5], which determines a high risk of significant blood loss after injection without the ability to stop bleeding. At the same time, the authors reported 70% success.
In our protocol, we describe the catheterization of the mouse renal artery by inserting a thin catheter into a small puncture in the femoral artery and then approaching the left renal artery through the abdominal aorta [6–8]. This protocol is similar to the standard clinical approach [9–11]. It does not require blockade of the target kidney blood flow, disruption of the integrity, and ligation of the renal artery or abdominal aorta, unlike other described methods [1–4, 12, 13]. This protocol requires microsurgery skills but can be extremely useful for studies in which maintaining a normal blood supply to the target kidney as well as vascular integrity is critical.
Materials and reagents
Biological materials
Balb/c mice 8–10 weeks old (20–25 g weight) were obtained from the vivarium of the Saratov State Medical University. Other mouse strains could also be used in this protocol.
Reagents
Corneregel 5% (Dr. Gerhard Mann, catalog number: 4030571000075)
Zoletil 100 Virbac SA (Valdepharm / Delpharm Tours, catalog number: SAGARPA Q-0042-306)
Xyla (Xylazine 2%) 50 mL (Interchemie werken, catalog number: IX2)
Iodine alcohol solution for external use, alcohol 5% (Pharmaks, catalog number: 322645 or Rigas Farmfabrika, catalog number: 110004)
Sodium chloride solution (saline) for injection 0.9% 5 mL (Grotex LLC, catalog number: 250309 or Hameln, UK, catalog number: SC005)
Evans Blue (Sigma-Aldrich, catalog number: E2129)
70% alcohol antiseptic solution (BioPharmKombinat, catalog number: N1x1)
Solutions
5% Evans Blue solution in saline (see Recipes)
Recipes
5% Evans Blue solution in saline (1 mL)
Reagent Final concentration Quantity or Volume Evans Blue 5% (w/v) 5 mg Sodium chloride solution (0.9%) 0.9% (w/v) 1 mL Total (optional) 5% (w/v) 1 mL
Laboratory supplies
Nylon surgical threads (USP: 5/0, EP: 1) (Mosnitki, catalog number: 5/0(1))
Cotton pads, cleanic soft & сomfort (Harper Hygienics, catalog number: 96190075)
Cotton buds (Harper Hygienics, catalog number: 96190075)
Fixing adhesive plaster 2.5 cm × 5 m (Nordeplast, catalog number: 1145541)
Sterile surgical cotton wool 100 g (Hygrovata, catalog number: GOST 5556-81)
Hair removal cream for sensitive skin Veet (Reckitt Benckiser, catalog number: BP_29060)
Suture material Prolene 5-0 (Ethicon, Johnson & Johnson, catalog number: W8310)
BD Micro-Fine Plus Demi insulin syringes with sterile interior 0.3 mm (30G) × 8 mm, 0.3 mL (Becton Dickinson, catalog number: 320829)
Polyurethane intravascular tubing 32ga/0.8Fr, 0.13 mm × 0.25 mm (Instech Laboratories, Inc., catalog number: BTPU-010)
Polyethylene tubing PE-10, 0.28 mm × 0.61 mm (BD Intramedic, catalog number: 22-204008)
Equipment
Dressing forceps 10 cm (Figure 1A)
Tweezers 11 cm (Figure 1B)
Angle tweezers 10.5 cm (Figure 1C)
Toothed tweezers 14 cm (Figure 1D)
Tweezers 14 cm (Figure 1E)
Micro scissors 14 cm (Figure 1F)
Microneedle holder 14 cm (Figure 1G)
Stereomicroscope (Nexcope, model: NSZ-608)
LED ring light (Lomo-microsystems, model: LED-144A)
Digital camera MS-8.3 USB 3.0 with matrix 1/1.2" SONY (Lomo-microsystems, model: MS-8.3)
Analytical balance (OHAUS, model: Pioneer PR224)
Trimmer Wahl Micro Lithium (Wahl GhbH, model: 5640)
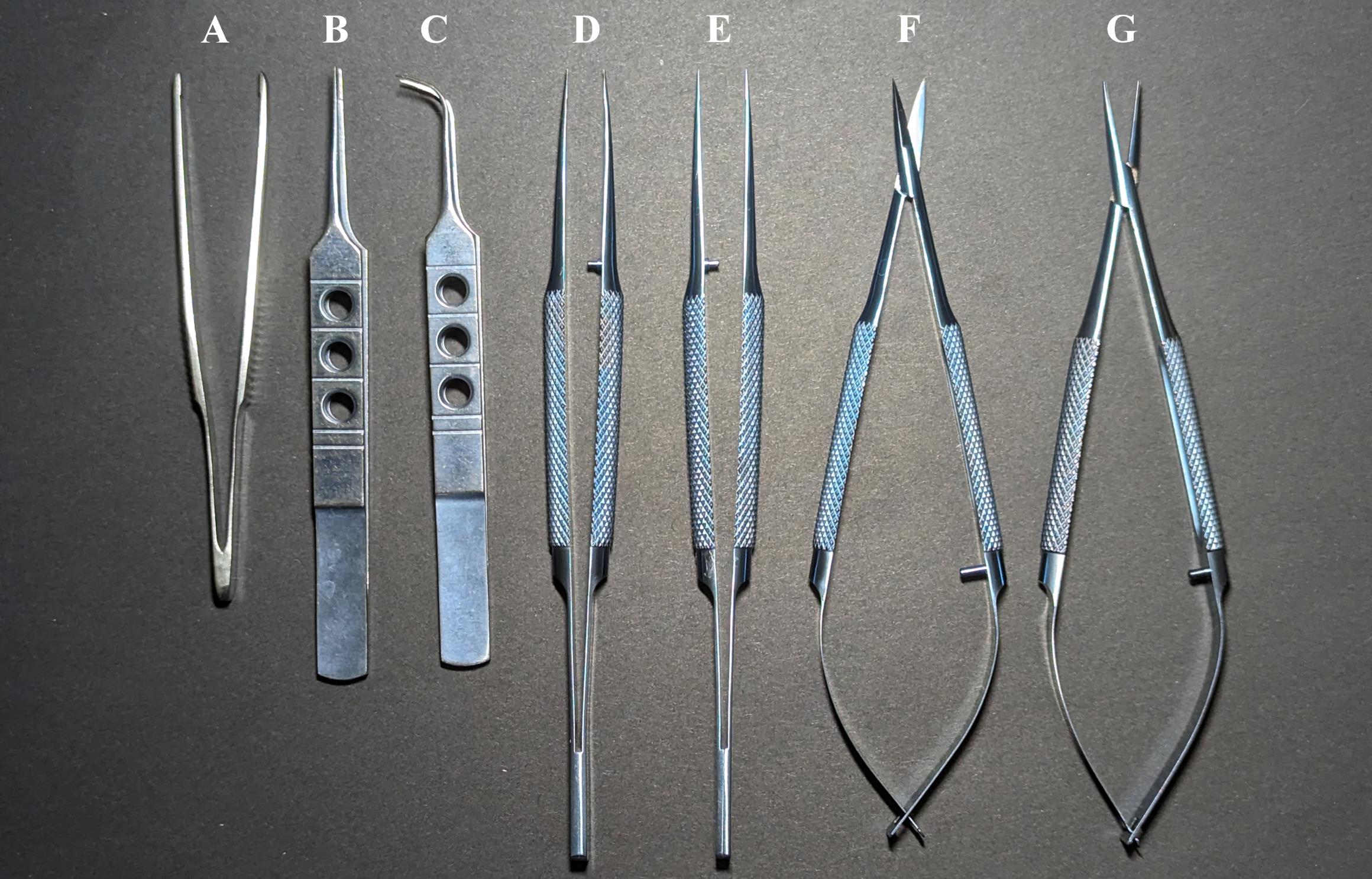
Figure 1. Catheterization tools. Dressing forceps 10 cm (A), tweezers 11 cm (B), angle tweezers 10.5 cm (C), toothed tweezers 14 cm (D), tweezers 14 cm (E), micro scissors 14 cm (F), and microneedle holder 14 cm (G).
Software and datasets
For video recording: Pylon Viewer (6.3.0.23157, 02.11.2021)
For video processing: Shotcut (24.02.29, 29.02.2024)
Procedure
文章信息
稿件历史记录
提交日期: Mar 31, 2024
接收日期: Jul 29, 2024
在线发布日期: Aug 16, 2024
出版日期: Sep 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Sindeeva, O. A., Gusliakova, O. I., Prikhozhdenko, E. S., Shushunova, N. A. and Sukhorukov, G. B. (2024). Mouse Renal Artery Catheterization for Local Delivery of Drugs in Capsulated or Free Forms. Bio-protocol 14(18): e5070. DOI: 10.21769/BioProtoc.5070.
分类
医学
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link