- EN - English
- CN - 中文
In Vitro GT-array (i-GT-ray), a Platform for Screening of Glycosyltransferase Activities and Protein–Protein Interactions
体外 GT 阵列 (i-GT-ray):筛选糖基转移酶活性和蛋白质相互作用的平台
发布: 2024年09月20日第14卷第18期 DOI: 10.21769/BioProtoc.5066 浏览次数: 2385
评审: David PaulWeidong AnAnonymous reviewer(s)

相关实验方案
基于蛋白筛选策略从合成文库中分离抗原特异性纳米抗体:结合 MACS 的酵母展示筛选与 FLI-TRAP 方法
Apisitt Thaiprayoon [...] Dujduan Waraho-Zhmayev
2026年01月20日 410 阅读
Abstract
Progress in bioinformatics has facilitated the identification of a large number of putative glycosyltransferases (GTs) associated with many physiological processes. However, many of these GTs remain with unknown biochemical function due to numerous technical limitations. One of these limitations is the lack of innovative tools for large-scale screening of enzyme activity in vitro and testing protein–protein interactions (PPIs) between GT partners. Currently, testing the enzyme activity of a protein requires its production in a heterologous expression system and purification before enzyme assays, a process that is time-consuming and not amenable to high-throughput screening. To overcome this, we developed a platform called in vitro GT-array (i-GT-ray). In this platform, 96-well microplates are coated with plasmid DNA encoding for tagged GTs and a capture antibody. Tagged GTs are produced from plasmid DNA via a cell-free in vitro transcription/translation (IVTT) system and captured through the anti-tag capture antibody directly on microplates. After washing to remove IVTT components, the captured enzymes can be considered purified, and their activity can be tested directly on microplates. The whole process can be performed in less than two days, compared to several weeks for currently available screening methods. The i-GT-ray platform has also been adapted to investigate PPIs between GTs. Here, we provide a practical user guide for the preparation of GT-arrays coated with plasmid DNA and a capture antibody that can be used for monitoring enzyme activity and PPIs of GTs in a high-throughput manner.
Key features
• Synthesis of tagged proteins directly from plasmid DNA, which are captured by anti-tag antibody attached to microplates.
• Captured tagged proteins can be considered as purified proteins ready for enzyme assays.
• Our platform can be used for high-throughput screening of enzyme activity and protein–protein interactions in vitro in a short time.
• Our platform can be used for biochemical characterization of difficult proteins such as membrane-integrated glycosyltransferases.
• Our platform can be adapted to downstream analytical methods such as mass spectrometry (i.e., DPS-MS).
Keywords: Protein synthesis (蛋白质合成)Graphical overview
Background
Advancements in high-throughput genomics technologies, including DNA sequencing, DNA microarrays, RNA-Seq, and proteomics, have greatly facilitated the identification of numerous genes across various species. However, methods for high-throughput biochemical analyses of proteins encoded by these genes are lacking [1]. Determining the enzyme activity of a protein can be achieved through direct testing in vitro or indirectly via genetic complementation of mutants, which are poorly adapted for high throughput. Currently, characterizing the biochemical function of an enzyme involves expression in heterologous organisms that typically lack that protein, followed by measurement of enzyme activity of solubilized and partially purified protein [2–4]. This approach has several drawbacks, including high cost, low yield, time consumption, and limited applicability to high-throughput analyses.
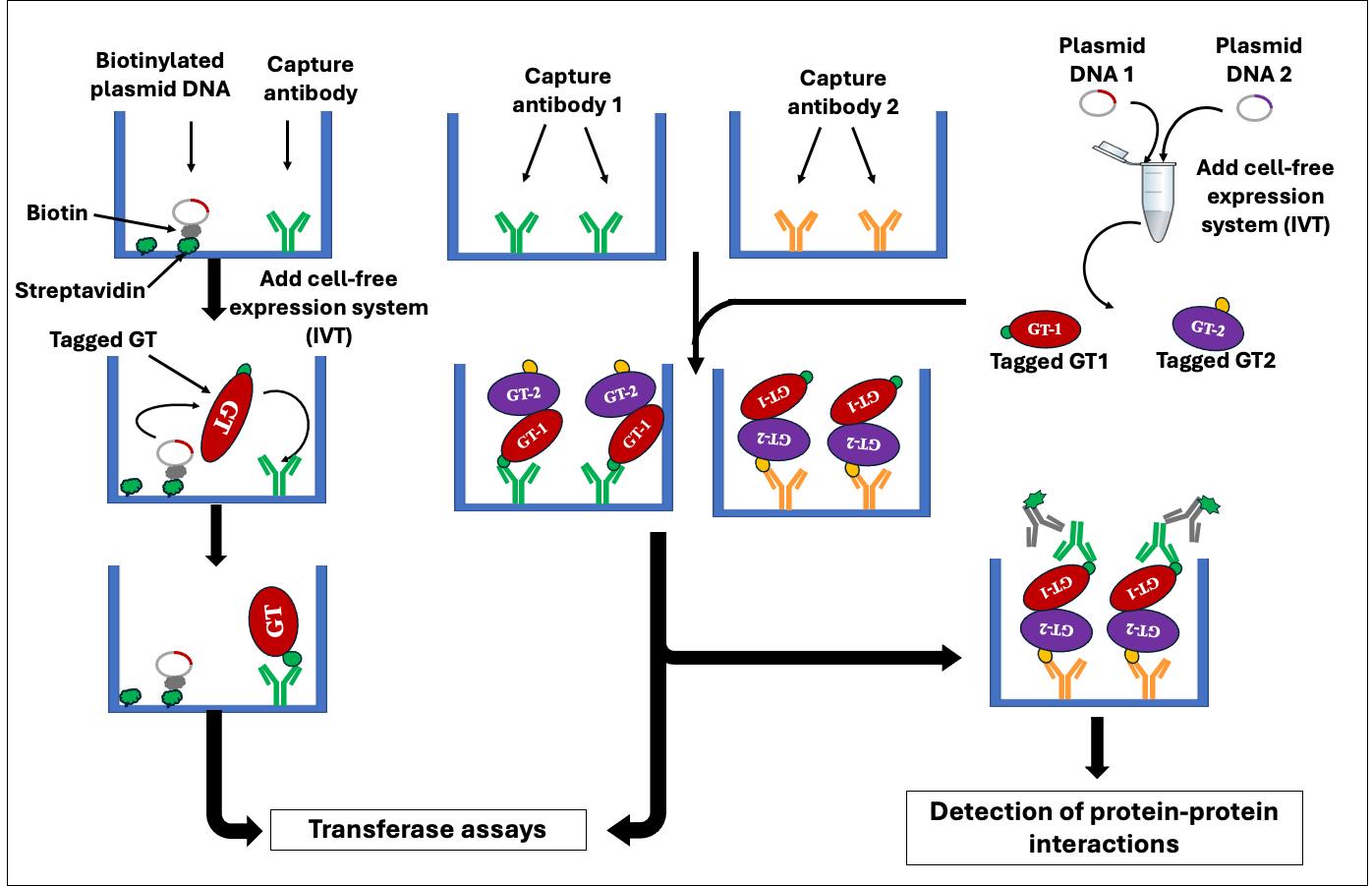
The development of protein microarrays requires the production of substantial quantities of purified proteins for binding onto a solid support and necessitates specialized instrumentation, such as microarray printers and scanners [5–7]. A recent advancement in protein microarray technology, called nucleic acid programmable protein array (NAPPA), has facilitated the efficient generation of protein microarrays through in vitro cell-free transcription/translation (IVTT) systems directly from plasmid DNA [8]. Currently, NAPPA is primarily employed in screening for protein–protein interactions (PPIs) [5,9–12]. We have recently developed and validated a NAPPA-based platform for enzyme assays of glycosyltransferases (GTs) named in vitro GT-array (i-GT-ray) [13,14]. Additionally, it enables the study of PPIs of GTs [15] in a high-throughput manner. Most eukaryotic GTs are difficult to characterize biochemically through conventional methods because they are integral membrane proteins [16] and are present (along with their corresponding mRNAs) in very low quantities within the cell. These characteristics pose challenges in production and purification [16,17]. Interestingly, in this platform, all GTs tested during validation work could be produced in a soluble and active form even though the cell-free coupled IVTT systems do not contain any lipids or detergent (membrane mimic). The i-GT-ray platform utilizes 96-well microplates pre-coated with plasmid DNA-encoding tagged proteins and anti-tag capture antibody (CAb). This enables simultaneous protein production using IVTT systems and protein capturing via the CAb on microplates (Figure 1A and B). Following washing, the captured GTs can be screened for enzyme activity directly on microplates (Figure 1C). The products resulting from transferase reactions can be detected using mass spectrometry or the GLO system directly on microplates (Figure 1D). This unique platform facilitates the rapid and effective creation of GT arrays, significantly reducing the time required for enzyme activity screening. In this manuscript, we provide a step-by-step protocol for the preparation of GT arrays, which can be utilized for in vitro enzyme activity screening and PPIs of GTs. The potential pitfalls of using full-length membrane integral proteins are discussed.
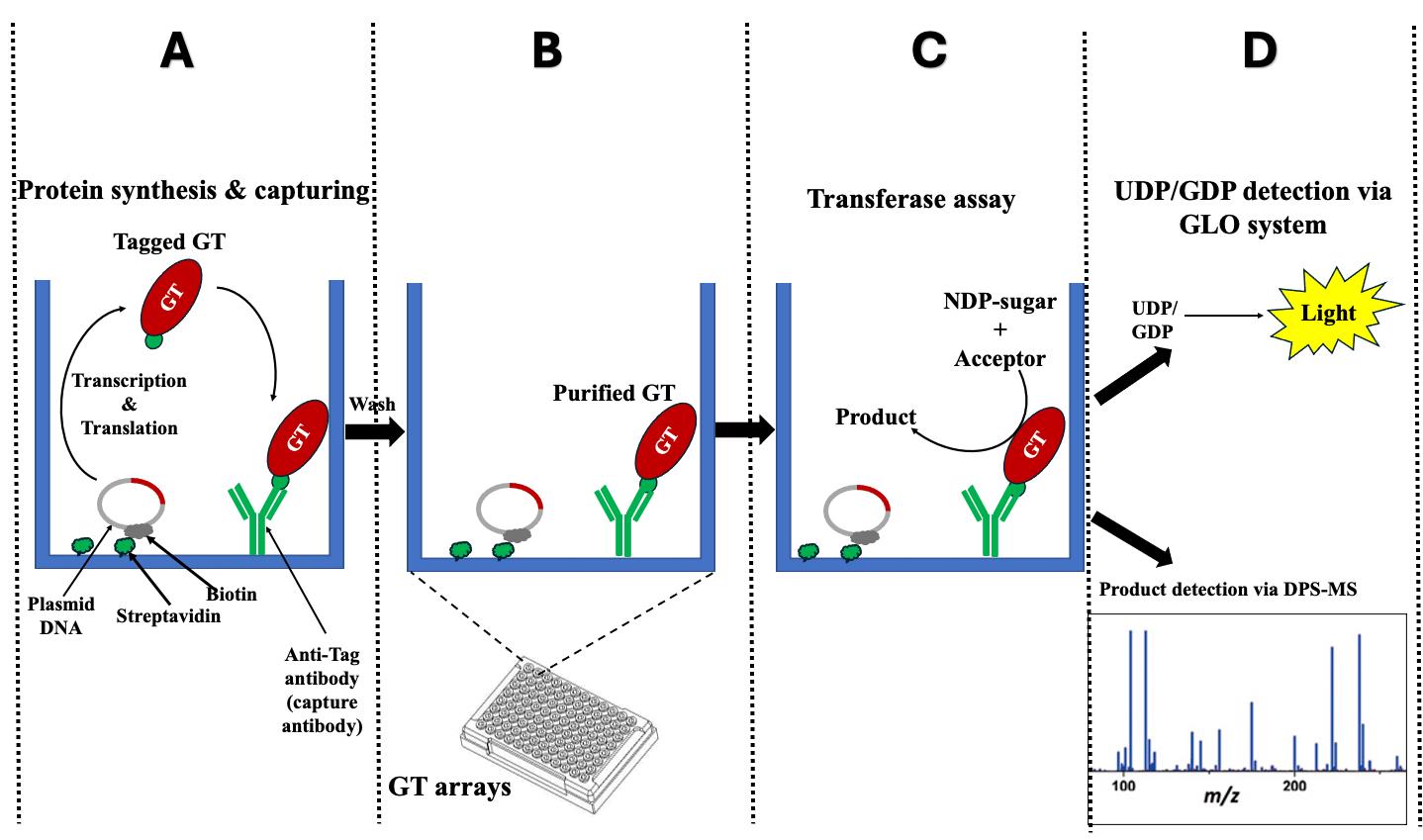
Figure 1. Schematic representation illustrates the fundamental principle of the i-GT-ray platform. (A) The tagged glycosyltransferases (GTs) are simultaneously synthesized and attached on microplate wells using plasmid DNA and anti-tag capture antibody. (B) After washing, the captured tagged GTs are considered purified. (C) Transferase reactions can be carried out by adding nucleotide diphosphate (NDP) sugars and acceptors. (D) The monitoring of transferase reactions is achieved through various methods, including the UDP/GDP GLO system, radioactive assays, or DPS-MS.
Materials and reagents
Biological materials
Chemically competent E. coli cloning strain (e.g., One ShotTM TOP10 Chemically Competent E. coli, Invitrogen, catalog number: C404010 or equivalent)
Expression vector pJFT7_nHALO_DC (Ampicillin resistant, DNASU Plasmid Repository, Clone ID: EvNO000424503) and pANT7_cGST (Ampicillin resistant, DNASU Plasmid Repository, Clone ID: EvNO00023103)
GatewayTM donor vector (non-ampicillin resistant, e.g., pCRTM8/GW/TOPOTM TA, Fisher Scientific, catalog number: K250020)
LR ClonaseTM II enzyme mix (Invitrogen, catalog number: 11791-020)
High-fidelity polymerase (e.g., KAPA HiFi DNA Polymerase, Roche Sequencing and Life Science, catalog number: KK2102)
Taq DNA polymerase (New England Biolabs, catalog number: M0273L)
Template for full-length protein-coding sequences (CDS) of interest or a CDS clone
1-Step Human Coupled IVT Kit, DNA (Thermo Scientific, catalog number: 88882)
TnT® Quick Coupled Transcription/Translation System (Promega, catalog number: L1170)
Anti-GST antibody (Cytiva, catalog number: 27457701)
Anti-HaloTag® pAb (Promega, catalog number: G9281)
Secondary mouse anti-goat IgG HRP conjugated (Santa Cruz Biotechnology, catalog number: sc-2354)
Secondary anti-rabbit IgG HRP conjugated (Promega, catalog number: W4011)
PfoI restriction enzyme (Thermo Scientific, catalog number: ER1751)
Streptavidin (Thermo Scientific, catalog number: 434302)
Reagents
Gel-purification kit (Zymo Research, catalog number: D4007)
DNA Clean and concentratorTM (Zymo Research, catalog number: D4004)
LB broth (Fisher Scientific, catalog number: BP1426-2)
Agar (Research Products International, catalog number: A20020-100.0)
Agarose ME (Research Products International, catalog number: A20085-500.0)
Ampicillin (Sigma-Aldrich, CAS number: 69-52-3)
Spectinomycin (Research Products International Corp., catalog number: 22189-32-8)
NucleoBond Xtra Midi kit (MACHEREY-NAGEL, catalog number: 740420.10)
2× Laemmli sample buffer (Bio-Rad, catalog number: 1610737EDU)
SuperSignalTM West Femto maximum sensitivity substrate (Thermo Scientific, catalog number: 34096)
Tween-20 (Sigma-Aldrich, CAS number: 9005-64-5)
EDC [1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride] (Thermo Scientific, catalog number: 22980)
EZ-LinkTM Hydrazide-Biotin (Thermo Scientific, catalog number: 21339)
Non-fat dried milk bovine (Sigma-Aldrich, catalog number: M7409-1BTL)
Acrylamide/bis 37.5:1 (Bio-Rad, catalog number: 1610158)
Ammonium persulfate (APS) (Sigma-Aldrich, catalog number: 7727-54-0)
TEMED (Bio-Rad, catalog number: 1610801)
Imidazole (Sigma-Aldrich, CAS number: 288-32-4)
ZebaTM Micro Spin desalting columns, 7 K MWCO (Thermo Scientific, catalog number: 89877)
AccuBlue Next Gen dsDNA Quantitation kit (Biotium, catalog number: 31060-T)
UDP-GLO Kit (UDP-GloTM Glycosyltransferase Assay) (Promega, catalog number: V6961)
GDP-GLO Kit (GDP-GloTM Glycosyltransferase Assay) (Promega, catalog number: VA1090)
DOWEX 1X8-100 resin (Cl) (Fisher Scientific, catalog number: AAL142570B)
Ethanol (Fisher Chemical, catalog number: A962-4)
ScintiVerseTM BD Cocktail (ScintanalyzedTM) (Fisher Chemical, catalog number: SX18-4)
Methanol (Fisher Scientific, catalog number: A434-20)
Tris-HCL (Sigma-Aldrich, catalog number: 1185-53-1)
Tris-Base (Sigma-Aldrich, catalog number: 77-86-1)
EDTA (Sigma-Aldrich, catalog number: 60-00-4)
Glycine (Bio-Rad, catalog number: 1610718)
HCl (Fisher Scientific, catalog number: A144-50)
SDS (Vivantis, catalog number: 151-21-3)
NaCl (Sigma-Aldrich, catalog number: 7647-14-5)
KCl (Sigma-Aldrich, catalog number: 7447-40-7)
NaOH pellets (Fisher Scientific, catalog number: S318-100)
Na2HPO4 (Fisher Scientific, catalog number: P285-500)
NaH2PO4 (Fisher Scientific, catalog number: S381-500)
KH2PO4 (Fisher Scientific, catalog number: S375-212)
Na2CO3 (Sigma-Aldrich, catalog number: 497-19-8)
NaHCO3 (Sigma-Aldrich, catalog number: 144-55-8)
DMSO (Sigma-Aldrich, catalog number: 67-68-5)
MgCl2 (Sigma-Aldrich, catalog number: 7786-30-3)
MnCl2 (Sigma-Aldrich, catalog number:7773-01-5)
Solutions
LB medium (1 L) (see Recipes)
100 mg/mL antibiotic stock (Ampicillin or Spectinomycin) (10 mL) (see Recipes)
LB agar plate with antibiotics (1 L) (see Recipes)
SDS-PAGE gel working solutions (see Recipes)
SDS-PAGE running buffer (1 L) (see Recipes)
SDS-PAGE transfer buffer (1 L) (see Recipes)
100 mM phosphate buffer pH 7.2 (1 L) (see Recipes)
10 mM phosphate buffer pH 7.2 (1× PBS) (1 L) (see Recipes)
10 mM phosphate buffer pH 7.2 (1× PBS) containing 5% (w/v) fat-free milk (100 mL) (see Recipes)
10 mM phosphate buffer pH 7.2 (1× PBS) containing 0.05% (v/v) Tween 20 (1 L) (see Recipes)
10 mM phosphate buffer pH 7.2 (1× PBS) containing 5% (w/v) fat-free milk and 0.05% (v/v) Tween 20 (100 mL) (see Recipes)
10 mM phosphate buffered saline with EDTA (PBS with EDTA) (1 L) (see Recipes)
Sodium bicarbonate buffer (pH 9.6) (see Recipes)
50 mM biotin hydrazide (1 mL) stock (see Recipes)
2.5 mM biotin hydrazide (1 mL) working solution (see Recipes)
100 mM Tris-HCl buffer (pH 7.2) (see Recipes)
Recipes
LB medium (1 L)
Reagent Final concentration Quantity LB broth 25 g/L 25 g ddH2O n/a ~980 mL Total n/a 1,000 mL Adjust the pH to 7.4 with NaOH. Autoclave at 121 °C for 15 min. Store at RT.
100 mg/mL antibiotic stock (ampicillin or spectinomycin)
Reagent Final concentration Quantity Ampicillin or Spectinomycin 100 mg/mL 1 g ddH2O n/a 10 mL Total n/a 10 mL Sterilize the solution with a 0.2 μm syringe filter. Store at -20 °C.
LB agar plate with antibiotics (1 L)
Reagent Final concentration Quantity LB broth 25 g/L 25 g Agar 15 g/L 15 g Antibiotic stock (100 mg/mL) 100 μg/mL 1 mL ddH2O n/a ~960 mL Total n/a 1,000 mL Adjust the pH to 7.4 with NaOH. Add agar after adjusting pH. Autoclave at 121 °C for 15 min. Allow to cool to 35–45 °C. Add 1 mL of 100 mg/mL antibiotic solution and mix well. Transfer 25 mL of the solution to petri dishes. Allow the agar plates to cool and dry in a laminar flow hood. Store the plates at 4 °C.
SDS-PAGE gel working solutions (for three 1.0 mm gels)
Separation gel (10%)
Reagent Quantity ddH2O 6.7 mL 30% acrylamide/bis 37.5:1 5.3 mL Lower solution (1.5 M Tris-HCl pH 8.8 and 0.2% SDS) 4 mL Persulfate (10% Sol) 150 μL TEMED 10 μL Stacking gel
Reagent Quantity ddH2O 6 mL 30% acrylamide/bis 37.5:1 1.5 mL Upper solution (1 M Tris-HCl pH 6.8 and 0.2% SDS) 2.5 mL Persulfate (10% Sol) 40 μL TEMED 10 μL SDS-PAGE running buffer (1 L)
Reagent Final concentration Quantity Tris 30 mM 3.03 g Glycine 200 mM 14.5 g SDS 0.1% (w/v) 1 g ddH2O n/a ~980 mL Total n/a 1,000 mL Store at room temperature (RT).
SDS-PAGE transfer buffer (1 L)
Reagent Final concentration Quantity Tris 30 mM 2.525 g Glycine 200 mM 13.824 g ddH2O n/a ~780 mL Methanol 20% (w/v) 200 mL Total n/a 1,000 mL Adjust the pH to approximately 8.3 after adding Tris and glycine in ~780 mL of ddH2O. Then, add 200 mL of 100% methanol and add ddH2O to 1 L. Store buffer at RT.
100 mM phosphate buffer pH 7.2 (1 L)
Reagent Final concentration Quantity NaCl 1.36 M 80 g KCl 26.83 mM 2 g Na2HPO4 141.96 mM 14.4 g KH2PO4 17.64 mM 2.4 g ddH2O n/a ~900 mL Total n/a 1,000 mL Adjust pH to approximately 7.2. Autoclave at 121 °C for 35 min. Store at 4 °C.
10 mM phosphate buffer pH 7.2 (1× PBS) (1 L)
Reagent Final concentration Quantity 100 mM phosphate buffer pH 7.2 10 mM 100 mL ddH2O n/a ~900 mL Total n/a 1,000 mL Adjust pH to 7.2. Store buffer at 4 °C.
10 mM phosphate buffer pH 7.2 (1× PBS) containing 5% (w/v) fat-free milk (100 mL)
Reagent Final concentration Quantity 100 mM phosphate buffer pH 7.2 10 mM 10 mL Fat-free milk 5% 5 g ddH2O n/a ~90 mL Total n/a 1,00 mL Adjust pH to 7.2 for 1× PBS, add fat-free milk, and mix for 1 h. Store buffer at 4 °C.
10 mM phosphate buffer pH 7.2 (1× PBS) containing 0.05% (v/v) Tween 20 (1 L)
Reagent Final concentration Quantity 100 mM phosphate buffer pH 7.2 10 mM 100 mL Tween 20 0.05% (v/v) 0.5 mL ddH2O n/a ~900 mL Total n/a 1,000 mL Adjust pH to 7.2 for 1× PBS, add Tween 20, and mix for 1 h. Store buffer at 4 °C.
10 mM phosphate buffer pH 7.2 (1× PBS) containing 5% (w/v) fat-free milk and 0.05% (v/v) Tween 20 (100 mL)
Reagent Final concentration Quantity 100 mM phosphate buffer pH 7.2 10 mM 10 mL Tween 20 0.05% (v/v) 0.05 mL Fat-free milk 5% 5 g ddH2O n/a ~99 mL Total n/a 100 mL Adjust pH to 7.2 for 1× PBS, add Tween 20 and fat-free milk, and mix for 1 h. Store buffer at 4 °C.
10 mM phosphate buffered saline with EDTA (PBS with EDTA) (1 L)
Reagent Final concentration Quantity NaCl 150 mM 8.8 g EDTA (0.5 M, pH 8.0) 10 mM 20 mL Na2HPO4 6.7 mM 0.95 g NaH2PO4 3.34 mM 0.4 g ddH2O n/a ~950 mL Total n/a 1,000 mL Adjust the pH to approximately 7.2. Autoclave at 121 °C for 35 min. Store at 4 °C.
Sodium bicarbonate buffer (pH 9.6)
Reagent Final concentration Quantity Na2CO3 15 mM 1.59 g NaHCO3 34.87 mM 2.93 g ddH2O n/a ~900 mL Total n/a 1,000 mL Adjust the pH to approximately 9.6. Autoclave at 121 °C for 35 min. Store at 4 °C.
50 mM biotin hydrazide (1 mL) stock
Reagent Final concentration Quantity Hydrazide-Biotin 50 mM 12.917 mg DMSO n/a 1 mL Total n/a 1 mL Store the solution at -20 °C.
2.5 mM biotin hydrazide (1 mL) working solution
Reagent Final concentration Quantity Hydrazide-biotin (50 mM) 2.5 mM 5 mL 0.1 M imidazole, pH 6 n/a 995 mL Total n/a 1 mL 100 mM Tris-HCl buffer (pH 7.2)
Reagent Final concentration Quantity Tris-HCl 100 mM 12.14 g MgCl2 2 mM 0.19 g MnCl2 2 mM 0.252g ddH2O n/a ~900 mL Total n/a 1,000 mL Adjust the pH to approximately 7.2. Sterilize the solution by filtration with a 0.2 μm syringe filter. Store at 4 °C.
Laboratory supplies
Pipette tips 0.5–10 µL, 20–200 µL, 100–1,000 µL (Fisher Scientific, FisherbrandTM, catalog numbers: 02-707-438, 02-707-417, 02-707-403)
1.5 mL Eppendorf tubes (Fisher Scientific, FisherbrandTM, catalog number: 02-681-321)
2 mL Eppendorf tubes (Fisher Scientific, FisherbrandTM, catalog number: 02-681-320)
Liquid scintillation vials (DWK Life Sciences, catalog number: 986730)
0.2 mL PCR tubes (Fisher Scientific, Thermo ScientificTM, catalog number: AB-0620)
100 mm × 15 mm Petri dishes (Fisher Scientific, FisherbrandTM, catalog number: FB0875712)
14 mL culture tubes (Globe Scientific, catalog number: 110178)
0.2 μm sterile syringe filter (e.g., Corning, catalog number: 431227)
Immobilon membrane (Millipore Sigma, catalog number: IPFL85R)
96-well microplate (clear, flat bottom, half area, high binding, polystyrene) (Corning, catalog number: 3690)
96-well microplate (flat bottom, half area, non-binding, white polystyrene) (Corning, catalog number: 3642)
Whatman® cellulose chromatography papers (Whatman, catalog number: 3017-820)
Equipment
250 mL Erlenmeyer flask
Micropipettes 0.1–2.5 µL, 2–20 µL, 20–200 µL (Eppendorf, models: 3123000217, 3123000233, 3123000250)
Multichannel pipettes (8 channels) 0.1–10 µL, 10–100 µL (Eppendorf, models: 2231300043, 2231300045)
Benchtop microcentrifuge (Beckman Coulter, model: Microfuge 22R centrifuge, catalog number: 8043-30-1145)
PCR thermocycler (Applied biosystems, Veriti 96-well Thermal Cycler 2990215201, catalog number: 4375305)
Incubation shaker (New Brunswick Scientific, Classic series, model: M1247-0004, catalog number: 19525)
Incubator (Fisher Scientific, Thermo ScientificTM, model: HerathermTM IGS60, catalog number: 51028063)
Mini-PROTEAN Tetra vertical electrophoresis system (Bio-Rad, catalog number: 1658002EDU)
Gel electrophoresis tank (Bio-Rad Laboratories, model: Wide Mini-Sub®, catalog number: 1704468)
Gel electrophoresis power supply (Bio-Rad Laboratories, model: PowerPacTM Basic, catalog number: 1645050)
Gel imager (Bio-Rad Laboratories, model: Gel DocTM XR+, catalog number: 1708195)
Plate reader (BioTek Synergy/HTX multi-mode reader, model: S1LFA, catalog number: BTS1LFA)
ChemiDocTM imaging system (Bio-Rad Laboratories, Gel Doc EZ, catalog number: 1708270)
Multi-purpose scintillation counter (Beckman, model: LS6500, SKU: 8043-30-1194)
Freeze dry system (Labconco, model: 4.5 FreeZone, catalog number: 7750020)
Vortex
Water bath
Software and datasets
SnapGene Viewer v.7.1.1
Gen5 v.2.09.2
Microsoft® Excel V 16.86
Procedure
文章信息
稿件历史记录
提交日期: May 4, 2024
接收日期: Aug 1, 2024
在线发布日期: Aug 16, 2024
出版日期: Sep 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Bhattarai, M., Javaid, T., Venkataraghavan, A. and Faik, A. (2024). In Vitro GT-array (i-GT-ray), a Platform for Screening of Glycosyltransferase Activities and Protein–Protein Interactions. Bio-protocol 14(18): e5066. DOI: 10.21769/BioProtoc.5066.
- Bhattarai, M., Wang, Q., Javaid, T., Venkataraghavan, A., Al Hassan, M. T., O’Neill, M., Tan, L., Chen, H. and Faik, A. (2024). Streamlining assays of glycosyltransferases activity using in vitro GT-array (i-GT-ray) platform: Application to family GT37 fucosyltransferases. J Biol Chem. 300(3): 105734.
分类
生物化学 > 蛋白质
分子生物学 > 蛋白质 > 蛋白质-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link