- EN - English
- CN - 中文
In Vitro Hyphal Branching Assay Using Rhizophagus irregularis
利用Rhizophagus irregularis进行体外菌丝分枝实验
发布: 2024年08月20日第14卷第16期 DOI: 10.21769/BioProtoc.5054 浏览次数: 2310
评审: Andrea GramaticaRaju MondalAnonymous reviewer(s)
Abstract
Most terrestrial plants are associated with symbiotic Glomeromycotina fungi, commonly known as arbuscular mycorrhizal (AM) fungi. AM fungi increase plant biomass in phosphate-depleted conditions by allocating mineral nutrients to the host; therefore, host roots actively exude various specialized metabolites and orchestrate symbiotic partners. The hyphal branching activity induced by strigolactones (SLs), a category of plant hormones, was previously discovered using an in vitro assay system. For this bioassay, AM fungi of the Gigaspora genus (Gigasporaeae) are commonly used due to their linear hyphal elongation and because the simple branching pattern is convenient for microscopic observation. However, many researchers have also used Glomeraceae fungi, such as Rhizophagus species, as the symbiotic partner of host plants, although they often exhibit a complex hyphal branching pattern. Here, we describe a method to produce and quantify the hyphal branches of the popular model AM fungus Rhizophagus irregularis. In this system, R. irregularis spores are sandwiched between gels, and chemicals of interest are diffused from the surface of the gel to the germinating spores. This method enables the positive effect of a synthetic SL on R. irregularis hyphal branching to be reproduced. This method could thus be useful to quantify the physiological effects of synthesized chemicals or plant-derived specialized metabolites on R. irregularis.
Key features
• Development of an in vitro hyphal branching assay using germinating spores of Rhizophagus irregularis.
• This in vitro assay system builds upon a method developed by Kameoka et al. [1] but modified to make it more applicable to hydrophilic compounds.
• Optimized for R. irregularis to count the hyphal branches.
• This bioassay requires at least 12 days to be done.
Keywords: AM symbiosis (丛枝菌根共生)Graphical overview
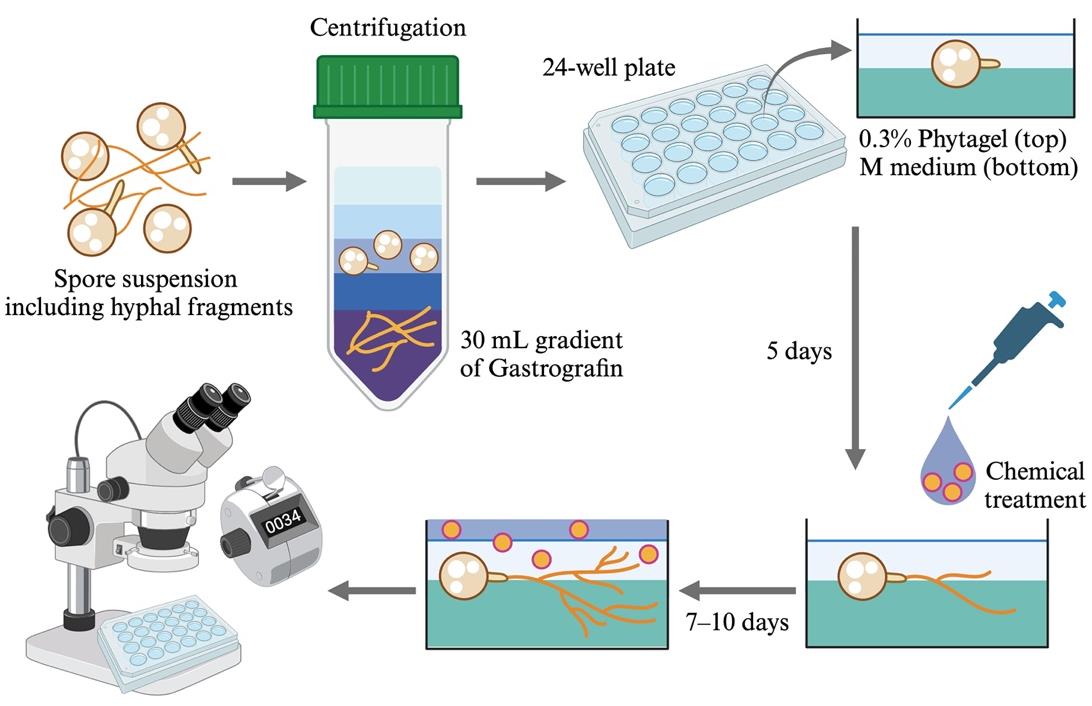
Simplified overview of the hyphal branching assay using Rhizophagus irregularis spores
Background
In nature, plants are surrounded by diverse and numerous microbes in their leaves (phyllosphere) and roots (rhizosphere). Since these microbes beneficially or detrimentally modulate the host’s growth, host plants utilize their own specialized metabolites to manipulate the assembly of microbes. In particular, Glomeromycotina fungi, termed arbuscular mycorrhizal (AM) fungi, are representative beneficial microbes that have been found to be associated with more than 70% of terrestrial plant species [2]. AM fungi allocate inorganic phosphate from the soil to the host plant and, in return, the host plant shares its photosynthates with the symbiotic fungi. This mutual interaction begins with a category of root-secreted plant hormones, the strigolactones (SLs), which possess hyphal branching activity in AM fungi [3]. SLs were initially identified from cotton root exudates and described as germination stimulants of root parasitic plants [4]. Forty years later, their hyphal branching activity was reported using an AM fungus of the Gigaspora genus, Gigaspora margarita [3,5]. Prior to that discovery, Gigaspora fungi had been used in bioassays to identify signal compounds in host root exudates [6–9]. Thus, in vitro assay systems using AM fungi are essential to clarify the chemical communication between AM fungi and host plants.
G. margarita usually forms large spores (approximately 200 μm in diameter), as suggested by the genus name [10]. In addition to a uniquely large spore size, the fungus exhibits a simple hyphal branching pattern, forming thick and linear hyphae [3,5]. Therefore, G. margarita has often been used for in vitro bioassays. However, recent studies aimed at revealing the molecular mechanisms regulating AM symbiosis have mainly applied Rhizophagus irregularis because fungal genomic information, including of several intraspecies lines, is available [11–13]. Another reason is that Rhizophagus fungi have more beneficial traits (i.e., plant growth promotion) than Gigaspora fungi [14,15]. On the other hand, Rhizophagus fungi have small spores (approximately 50 μm in diameter), thin and winding hyphae, and a complex branching pattern [16–19]. Taken together, the hyphal branching assay using R. irregularis is technically difficult [20]. This is the reason why bioassay using R. irregularis is performed by measuring the total length of hyphae [20–22].
In hyphal branching assays using Gigaspora fungi, chemicals of interest have been loaded onto paper discs [3,5]. On the other hand, diffusion assays are limited to the local treatment of reagents and require specialized techniques. In our previous study, we modified an in vitro bioassay system that was originally applied for asymbiotic sporulation of R. irregularis [1]. Using our protocol, which is technically easy, chemicals can be evenly applied to all parts of R. irregularis. Compared to the protocols for Gigaspora fungi, this system is useful for assessing the hyphal branching activity induced by various chemicals in this model AM fungus.
Materials and reagents
Biological materials
Rhizophagus irregularis DAOM197198 (4,000 spores/mL) (PremierTech)
Reagents
Acetone (FUJIFILM WAKO Pure Chemical, CAS number: 67-64-1)
Ethanol (99.5%) (FUJIFILM WAKO Pure Chemical, CAS number: 64-17-5)
Gastrografin for oral/enema use (Bayer, 597.3 g/L amidotrizoic acid, CAS number: 117-96-4)
Magnesium sulfate heptahydrate (MgSO4·7H2O) (FUJIFILM WAKO Pure Chemical, CAS number: 10034-99-8)
Potassium nitrate (KNO3) (FUJIFILM WAKO Pure Chemical, CAS number: 7757-79-1)
Potassium chloride (KCl) (FUJIFILM WAKO Pure Chemical, CAS number: 7447-40-7)
Potassium dihydrogen phosphate (KH2PO4) (FUJIFILM WAKO Pure Chemical, CAS number: 7778-77-0)
Calcium nitrate tetrahydrate [Ca(NO3)2·4H2O] (FUJIFILM WAKO Pure Chemical, CAS number: 13477-34-4)
Sucrose (FUJIFILM, CAS number: 57-50-1)
Fe(III)-EDTA (NaFeEDTA·3H2O) (DOJINDO, CAS number: 15708-41-5)
Potassium iodide (KI) (FUJIFILM WAKO Pure Chemical, CAS number: 7681-11-0)
Manganese chloride tetrahydrate (MnCl2·4H2O) (FUJIFILM WAKO Pure Chemical, CAS number: 13446-34-9)
Zinc sulfate heptahydrate (ZnSO4·7H2O) (FUJIFILM WAKO Pure Chemical, CAS number: 7446-20-0)
Boric acid (H3BO3) (FUJIFILM WAKO Pure Chemical, CAS number: 10043-35-3)
Copper sulfate pentahydrate (CuSO4·5H2O) (FUJIFILM WAKO Pure Chemical, CAS number: 7758-98-7)
Disodium molybdate(VI) dihydrate (Na2MoO4·2H2O) (FUJIFILM WAKO Pure Chemical, CAS number: 10102-40-6)
Potassium hydroxide (KOH) (FUJIFILM WAKO Pure Chemical, CAS number: 1310-58-3)
Glycine (FUJIFILM WAKO Pure Chemical, CAS number: 56-40-6)
Thiamine hydrochloride (FUJIFILM WAKO Pure Chemical, CAS number: 67-03-8)
Pyridoxine hydrochloride (FUJIFILM WAKO Pure Chemical, CAS number: 58-56-0)
Nicotinic acid (FUJIFILM WAKO Pure Chemical, CAS number: 59-67-6)
Myo-inositol (FUJIFILM WAKO Pure Chemical, CAS number: 87-89-8)
Phytagel (Sigma-Aldrich, CAS number: 71010-52-1)
rac-GR24 (StrigoLab, CAS number: 76974-79-3)
Solutions
M medium (see Recipes) [16]
Calcium stock of M medium (see Recipes)
1,000× vitamin stock of M medium (see Recipes)
0.3% phytagel containing 3 mM MgSO4·7H2O (see Recipes)
Recipes
M medium
Reagent Final concentration Quantity or Volume MgSO4·7H2O 2.97 mM 731 mg KNO3 0.79 mM 80 mg KCl 0.87 mM 65 mg KH2PO4 0.035 mM 4.8 mg Sucrose 10% (w/v) 10,000 mg NaFeEDTA·3H2O 0.022 mM 9.18 mg KI 0.0045 mM 0.75 mg MnCl2·4H2O 0.030 mM 6 mg ZnSO4·7H2O 0.0092 mM 2.65 mg H3BO3 0.024 mM 1.5 mg CuSO4·5H2O 0.00052 mM 0.13 mg Na2MoO4·2H2O 9.92 nM 0.0024 mg Phytagel 4% (w/v) 4,000 mg H2O n/a 1,000 mL Total (optional) n/a 1,000 mL Note: Adjust pH at 5.5 using KOH solution and autoclave at 121 °C for 20 min. n/a, not applicable.
Calcium stock of M medium
Reagent Final concentration Quantity or Volume Ca(NO3)2·4H2O 1.22 M 2,880 mg H2O n/a 10 mL Total (optional) n/a 10 mL Note: Sterilize the stock using a 0.45 μm filter and store at room temperature (23–25 °C) until use. n/a, not applicable.
1,000× vitamin stock of M medium
Reagent Quantity or Volume Glycine 30 mg Thiamine hydrochloride 1 mg Pyridoxine hydrochloride 1 mg Nicotinic acid 5 mg Myo-inositol 500 mg H2O 10 mL Total (optional) 10 mL Note: Sterilize the mixture using a 0.45 μm filter and store at -30 °C until use.
0.3% phytagel containing 3 mM MgSO4·7H2O
Reagent Final concentration Quantity or Volume MgSO4·7H2O 3 mM 739.4 mg Phytagel 0.3% (w/v) 3,000 mg H2O n/a 1,000 mL Total (optional) n/a 1,000 mL Note: No need to adjust pH. Autoclave at 121 °C for 20 min. n/a, not applicable.
Laboratory supplies
24-well plates (TrueLine, catalog number: TR5002)
1.5 mL microcentrifuge tube (BIO-BIK, catalog number: CF-0150)
15 mL centrifuge tube (Labcon, catalog number: 3132-345)
50 mL centrifuge tube (Labcon, catalog number: 3181-345)
10 mL disposable serological pipette (ASONE, catalog number: 2-4131-14)
Cell strainer (40 μm mesh) (Falcon, catalog number: 352340)
1 mL needleless plastic syringe (TERUMO, catalog number: SS-01T)
0.45 μm PTFE filter (Shimadzu, catalog number: GLCTD-HPTFE1345)
Inspection film tape (aglis, catalog number: LP0002K)
Equipment
Electronic pipette (Labnet, model: FASTPETTE Pro)
Laminar flow hood (SANYO, model: MCV-B131S)
Centrifuge (KUBOTA, model: 3740)
Swing rotor for 50 mL centrifuge tubes (KUBOTA, model: SD-242)
Autoclave (TOMY SEIKO, model: LSX-300)
Vacuum concentrator (Thermo Fisher Scientific, model: Savant SpeedVac DNA130)
Plant growth chamber (NIPPON MEDICAL & CHEMICAL INSTRUMENTS, model: LH-411S)
Stereomicroscope (Olympus, model: SZX16) equipped with a digital camera (Olympus, model: DP-26)
Imaging software (Olympus, model: CellSens standard v1.18)
Software and datasets
Microsoft Office Excel 10
R v4.2.0 (https://www.r-project.org/)
Procedure
文章信息
稿件历史记录
提交日期: May 20, 2024
接收日期: Jul 14, 2024
在线发布日期: Jul 31, 2024
出版日期: Aug 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Tominaga, T. and Kaminaka, H. (2024). In Vitro Hyphal Branching Assay Using Rhizophagus irregularis. Bio-protocol 14(16): e5054. DOI: 10.21769/BioProtoc.5054.
- Tominaga, T., Ueno, K., Saito, H., Egusa, M., Yamaguchi, K., Shigenobu, S. and Kaminaka, H. (2023). Monoterpene glucosides in Eustoma grandiflorum roots promote hyphal branching in arbuscular mycorrhizal fungi. Plant Physiol. 193(4): 2677–2690.
分类
植物科学 > 植物生理学 > 胞内共生
生物科学 > 生物技术 > 微生物技术
微生物学 > 微生物-宿主相互作用 > 真菌
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link