- EN - English
- CN - 中文
A Social Stimulation Paradigm to Ameliorate Memory Deficit in Alzheimer's Disease
一种改善阿尔茨海默病记忆缺陷的社交刺激范式
发布: 2024年08月05日第14卷第15期 DOI: 10.21769/BioProtoc.5046 浏览次数: 1768
评审: Salim GasmiRachael E. HokensonAnonymous reviewer(s)
Abstract
Alzheimer's disease (AD) poses a global health threat, progressively robbing patients of their memory and cognitive abilities. While it is recognized that meaningful social contact can alleviate the symptoms of dementia in AD patients, the precise mechanisms by which social stimulation mitigates AD symptoms remain poorly understood. We found that social interaction with novel mice, also known as novel social, simulated meaningful socializing. Therefore, we developed the multiple novel social (MNS) stimulation paradigm to train AD model mice and found that MNS effectively alleviated cognitive deficits in AD mice. This discovery not only opens up a new avenue for investigating the relationship between social stimulation and Alzheimer's disease but also lays the groundwork for delving into the underlying mechanisms, thereby providing crucial theoretical support for developing novel strategies to treat Alzheimer's disease.
Key features
• Designing a new social stimulation method to simulate meaningful social interactions in daily life.
• The MNS stimulation protocol spans 14 days, with one novel mouse introduced to the subject mice each day.
• The subjects were 2.5-month-old FAD4T mice, simulating patients with mild cognitive impairment (MCI).
• Results of behavioral tests confirm the efficacy of MNS in reducing cognitive deficits in the AD model.
Keywords: Alzheimer's disease (阿尔茨海默病)Graphical overview
Background
Alzheimer’s disease (AD) is one of the most influential and common neurodegenerative diseases in the world. It also ranks as the fifth leading cause of mortality among elderly individuals worldwide [1,2]. Memory and cognitive decline are recognized as the most incapacitating aspects of Alzheimer's disease [3]. In recent years, there have been emerging drugs in clinical practice that show promise in alleviating the dementia symptoms associated with Alzheimer’s disease [4–6]. However, the precise effects and potential side effects of these medications on cognitive function and disease progression are still being closely monitored. Non-pharmacological therapies have also been increasingly recognized and valued. Non-pharmacological treatments, such as engaging in physical activities, cognitive exercises, social interactions, and managing vascular and metabolic risks, are integrated alongside drug therapies for Alzheimer's disease [2].
Despite the recognition of non-pharmacological interventions, there remains a research gap regarding the mechanisms underlying the benefits of social activities. Studies have shown that an enriched social network and frequent social contact can prevent dementia and increase patients' cognitive reserve [7–11]. Additionally, social isolation exacerbates anxiety and asymmetrical brain atrophy in Alzheimer's patients [12]. Conversely, complex social interactions can improve episodic memory, enhance brain reserve, and reduce the risk of dementia [13]. Although the benefits of social stimulation for Alzheimer's patients have been well established, the specific biological mechanisms remain unclear.
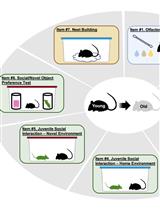
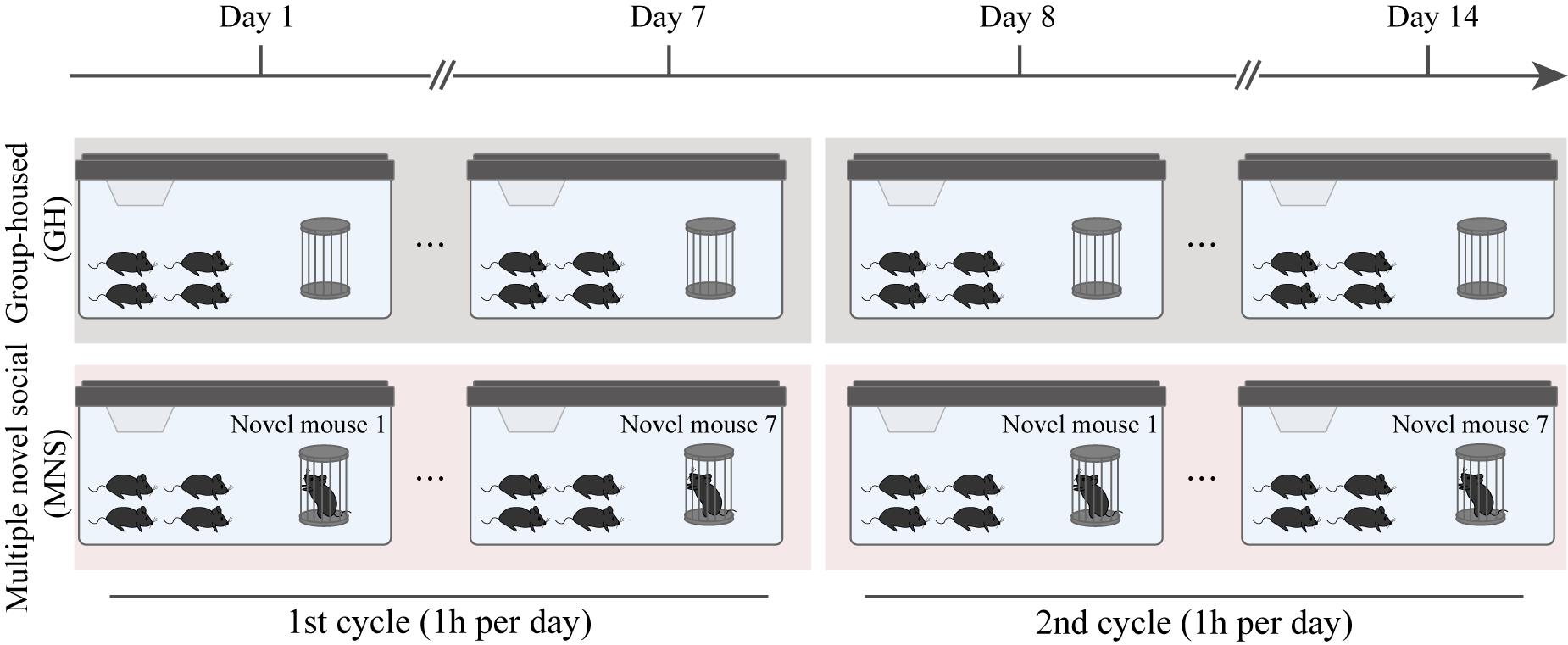
We found that a single novel social (SNS) stimulation with an unfamiliar mouse effectively activated the α-secretase activity in the ventral hippocampus of both wildtype (WT) and AD model mice and inhibited the amyloidogenic-cleavage pathway but was not enough to reduce the accumulation of Aβ in AD model mice [14]. Therefore, we implemented a 14-day multiple novel social (MNS) protocol to extend the intervention effect of this paradigm by increasing the number of social stimuli, allowing the subject mice to experience 14 novel social interactions. Given that the social memory of mice is transient, typically lasting only a few days [15], we cycled through seven different novel mice (designated N1–N7) for the first and second seven-day periods of the protocol. It should be noted that we also recommend using 14 different unfamiliar mice for the 14-day MNS protocol. As a control, group-housed (GH) subject mice were only able to interact with their familiar littermates.
After the MNS treatment, through the novel object recognition test and 3-chamber test, we found that the MNS stimulation paradigm significantly improved the cognitive ability and social memory of AD mice. Therefore, the MNS stimulation paradigm represents a promising method to alleviate dementia symptoms in Alzheimer’s disease.
Materials and reagents
Mice [all animals were housed in standard laboratory conditions with ad libitum access to food and water, as well as a 12/12 h light/dark cycle (light: 7:00 AM–7:00 PM), a temperature range of 22–26 °C, and a humidity level of 55%–60%. The mice used in this study were cared for in accordance with the authorized protocols of Southeast University, Nanjing, China)].
FAD4T mice [C57BL/6 genetic background, 10–12 weeks old. FAD4T mice co-expressed human APP with the Swedish (KM670/671NL) and India (V717F) variants, together with human mutant PS1 (M146L, L286V), driven by the mouse Thy1 promoter (Gempharmatech Co., Ltd, catalog number: T053302)]
Mice used for novel stimulation (N1–N7) and social test (S1–S2) (C57BL/6J genetic background, 10–12 weeks old, 22–25 g) (Gempharmatech Co., Ltd, catalog number: N000013, Jiangsu, China)
Nitrile laboratory gloves (Guangming, China)
Paper towels (Breeze, China)
75% Ethanol (Alladin, catalog number: A171299)
Equipment
Mouse breeding room with constant temperature system, ventilation system, and automatic light timing system
Home cage (380 mm × 180 mm × 170 mm, with a height under the partition bar inside the cage of 13 cm, which meets the requirements of the GB14925 national standard. It can accommodate 5–8 experimental mice weighing 20–30 g) (SHINVA, China)
Small electronic scale (127 mm × 106 mm × 19 mm) (Kubei, China)
Metal pen holder (golden, diameter 8 cm, height 10.3 cm, weight 140 g) (Wuling, China)
Timer (82 mm × 62 mm × 24 mm) (Anytime, model: XL-009B)
Spray bottle (500 mL) (LDPE, Sangon Biotech, catalog number: F505008)
Objects for novel object recognition (NOR) test (two bright-red metal cubes, 83 mm × 83 mm × 83 mm, were used as similar objects; one light-purple metal cylinder, 85 mm in diameter and 84 mm in height, was used as the novel object) (Aiziling, China)
Mouse behavioral testing room with constant temperature system, ventilation system, and automatic light timing system
Behavioral testing apparatus (Xinruan, China)
NOR apparatus
A square Plexiglas box (50 cm × 50 cm × 50 cm) with four 50 cm-high sidewalls and an open roof. In addition, two metal boxes with heavy objects inside were used as similar objects, which maintained consistency in size, color, and shape. Another metal box with a heavy object, similar in size but different in color and shape, was used as the novel object
3-chamber apparatus
A rectangular box made of Plexiglas (50 cm × 25 cm) with a detachable base, creating three distinct compartments divided by two partitions. Each partition contained a small door (10 cm × 5 cm) to facilitate mouse’s movement between the different chambers. In addition, there were two metal pen holders that were identical in size, shape, and color, used for restraining stranger mice.
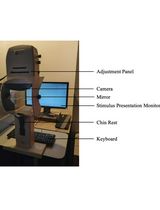
Computer (Lenovo, model number: Tianyi 510pro-14IMB)
Camera (MOKOSE, model number: HDC10)
Software and datasets
Video tracking and analyzing software (Noldus, EthoVision XT 13 software)
Statistical analysis software (Prism 8.0.1, GraphPad, https://www.graphpad.com/)
Procedure
文章信息
稿件历史记录
提交日期: May 2, 2024
接收日期: Jul 3, 2024
在线发布日期: Jul 19, 2024
出版日期: Aug 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ren, Q., Wang, S., Xie, W. and Liu, A. (2024). A Social Stimulation Paradigm to Ameliorate Memory Deficit in Alzheimer's Disease. Bio-protocol 14(15): e5046. DOI: 10.21769/BioProtoc.5046.
- Ren, Q., Wang, S., Li, J., Cao, K., Zhuang, M., Wu, M., Geng, J., Jia, Z., Xie, W., Liu, A., et al. (2024). Novel social stimulation ameliorates memory deficit in Alzheimer's disease model through activating α-secretase. J Neurosci.: e1689232024.
分类
神经科学 > 行为神经科学 > 认知
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link