- EN - English
- CN - 中文
Generation of Multicellular 3D Liver Organoids From Induced Pluripotent Stem Cells as a Tool for Modelling Liver Diseases
利用诱导多能干细胞生成多细胞三维肝类器官作为肝病模型的工具
发布: 2024年08月05日第14卷第15期 DOI: 10.21769/BioProtoc.5042 浏览次数: 2801
评审: Komuraiah MyakalaHsih-Yin TanAnonymous reviewer(s)
Abstract
The liver is an essential organ that is involved in the metabolism, synthesis, and secretion of serum proteins and detoxification of xenobiotic compounds and alcohol. Studies on liver diseases have largely relied on cancer-derived cell lines that have proven to be inferior due to the lack of drug-metabolising enzymes. Primary human hepatocytes are considered the gold-standard for evaluating drug metabolism. However, several factors such as lack of donors, high cost of cells, and loss of polarity of the cells have limited their widescale adoption and utility. Stem cells have emerged as an alternative source for liver cells that could be utilised for studying liver diseases, developmental biology, toxicology testing, and regenerative medicine. In this article, we describe in detail an optimised protocol for the generation of multicellular 3D liver organoids composed of hepatocytes, stellate cells, and Kupffer cells as a tractable robust model of the liver.
Key features
• Optimising a protocol for generating multicellular 3D liver organoids from induced pluripotent stem cells.
Keywords: Stem cells (干细胞)Graphical overview
Background
The liver is a vital organ that is involved in the metabolism, synthesis, and production of serum proteins and bile acid and the detoxification of xenobiotics [1]. Earlier liver models relied heavily on cancer-derived cell lines, which have been proven to be inferior due to the suboptimal activity of drug-metabolising enzymes [2,3]. Isolated primary human hepatocytes (PHH) are considered to be the “gold standard” for drug metabolism and toxicity screening [4,5]. However, these cells display a rapid decline in the phenotypic function when cultured in traditional two-dimensional monolayer cell cultures; also, there is a scarcity of donors [5]. To overcome these limitations of using PHHs, researchers have developed various approaches including genetic modification of the cells and three-dimensional cultures combined with tissue engineering and media compositions [1,6,7]. Additionally, advances in cell culture systems offer a great opportunity to generate patient-specific hepatocytes using 2D cell-based [2] and 3D organoid platforms [1,8].
The liver cells can be obtained by direct differentiation of iPSCs [7,9] and tissue-derived stem cells [1,6,10] by exploiting inductive and repressive signals essential for liver ontogenic development. The 3D cultured cells display better physiologic and metabolic features of the native liver tissue in comparison with 2D cell-based cultures [1,9]. However, the vast majority of the reported methods predominantly differentiate cells into hepatic epithelial lineage only, thus lacking essential supportive cellular lineages such as profibrotic hepatic stellate cells (hematopoietic stem cells; HSCs) and inflammatory cells (Kupffer cells; KCs). Hence, they lack the capacity to model inflammatory diseases [1,11]. To circumvent these challenges, other researchers developed co-culture cell models based on mixing both the epithelial cells and supportive lineages from iPSCs [12,13]. However, these methods suffer from artefactual inflammation and fibrosis resulting from the difficulty in choosing the appropriate culture medium and extracellular matrix in which multiple cell lineages can be co-maintained [12,13].
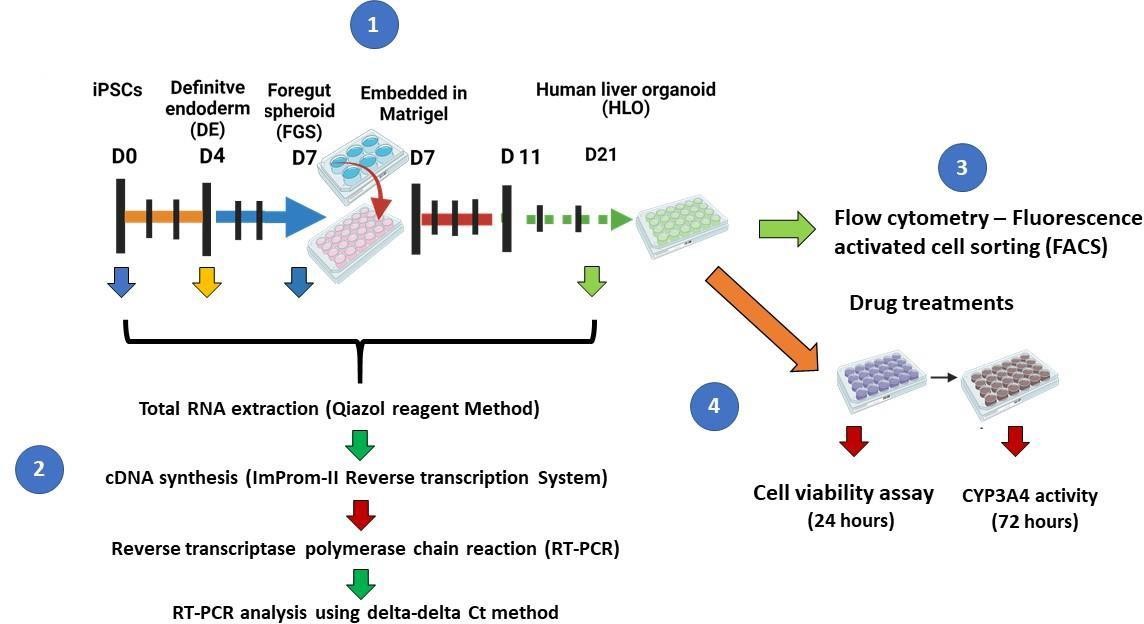
A recent study by Ouchi and colleagues described a protocol for the generation of a multicellular human liver organoid (HLO) composed of hepatocyte-like cells, hepatic stellate–like cells, and Kupffer-like cells from iPSCs [9]. The cells aggregated to form a 3D liver organoid that was used to model steatohepatitis after the addition of free fatty acids to the culture media [9]. In this paper, we describe a method for generating multicellular 3D liver organoids (Figure 1) adapted from the protocols published by Ouchi and colleagues [9] and Mun and colleagues [8]. We further describe in detail how to generate these organoids and demonstrate their utility as a tool to study downstream assays for liver disease models.
Materials and reagents
Biological materials
Schistosome eggs antigen (SEA) (Schistosome Biological Supply Center, Theodor Bilharz Research Institute)
Reagents
A83-01 (R&D Systems, catalog number: R2939)
Activin A (R&D Systems, catalog number: 338-AL)
Accutase (Sigma-Aldrich, catalog number: A6964)
CellTiter-GLO® cell viability assay (Promega, catalog number: PRG9681)
CHIR99021 (Sigma-Aldrich, catalog number: SML1046)
Advanced DMEM (Gibco, catalog number: 12634-010)
B27 supplement (Gibco, catalog number: A1486701)
Bone morphogenetic protein 4 (BMP-4) (R&D Systems, catalog number: 314-13P)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A8531)
Dexamethasone (Sigma-Aldrich, catalog number: D4902)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D2650)
Dulbecco’s phosphate buffered saline (DPBS) (Gibco, catalog number: 14190-094)
Essential 8 basal medium (Gibco, catalog number: A1517001)
Epidermal growth factor (R&D Systems, catalog number: 236-EG)
Ethylenediaminetetraacetic acid (EDTA) (Invitrogen, catalog number: 15575-020)
Fibroblast growth factor 4 (R&D Systems, catalog number: 325-4F)
Fibroblast growth factor 10 (R&D Systems, catalog number: F8924)
Geltrex (Life Technologies, catalog number: A1413302)
HCl (Merck, catalog number: 100317)
Hepatocyte culture medium (Lonza, catalog number: CC-3198)
Hepatocyte growth factor (Sigma-Aldrich, catalog number: H9661)
HEPES (Gibco, catalog number: 15630080)
ImProm-II reverse transcriptase (Promega, catalog number: PRA3800)
Knockout serum replacer (KSR) (Gibco, catalog number: 10828028)
N2 supplement (Gibco, catalog number: A1370701)
Oncostatin M (R&D Systems, catalog number: 295-OM)
P450-Glo CYP3A4 assay with Luciferin-IPA (Promega, catalog number: PRV9002)
Penicillin-streptomycin (Gibco, catalog number: 15140-12221)
PowerUPTM SYBRTM Green Master Mix (Applied Biosystems, catalog number: A25741)
Retinoic acid (RA) (Sigma-Aldrich, catalog number: R2625)
Rho kinase (ROCK) inhibitor (Y-27632) (Sigma-Aldrich, catalog number: Y0503)
RPMI-1640, GlutaMAX supplement (Gibco, catalog number: 61870-036)
TRIzolTM reagent (Thermo Fisher Scientific, catalog number: 15596026)
TryLETM Express enzyme (Thermo Fisher Scientific, catalog number: 12604013)
Vascular endothelial growth factor (R&D Systems, catalog number: 293-VE)
Vitronectin (Gibco, catalog number: A27940)
Antibody list for organoid phenotyping (Table 1)
Table 1. List of antibodies used to phenotype human liver organoids
Phenotype Antibody Concentration Fluorophore Host Vendor Dilution Epithelial cells EpCam 0.2 mg/mL BV421 Mouse BioLegend 1:80 Stellate cells CD166 5 µL (0.06 µg) PE-ALCAM Mouse eBioscience 1:160 Kupffer cells CD68 5 µL (0.05 µg) PE-Cy7 Mouse BioLegend 1:80 Antiretroviral and anti-tuberculosis drugs (Table 2)
Table 2. List of anti-retroviral and anti-tuberculosis drugs used to study liver injury
Drug Manufacturer Catalogue number Final concentration Efavirenz (EFV) Sigma-Aldrich 025M478 7.39 mM Tenofovir (TDF) Sigma-Aldrich SML1795 34.2 mM Lamivudine (3TC) Sigma-Aldrich L1295 27.91mM Rifampicin (RIF) Sigma-Aldrich R3501 28.42 mM Isoniazid (INH) Sigma-Aldrich MKCF2223 20.04 mM Primer sequences for RT-qPCR (Table 3)
Table 3. Primer sequences for stage-specific markers for liver organoids
Human stem cell markers Gene name Forward primer sequence (5'-3') Reverse primer sequence (5'-3') Oct-4 CAGGAGATATGCAAAGCAGAAAC GGCACTGCAGGAACAAATT Nanog AGCCTAATCAGCGAGGTTTC CAGAGCAAGACTCCGTTTCA Sox2 GCTACAGCATGATGCAGGACCA TCTGCGAGCTGGTCATGGAGTT Definitive endoderm (DE) markers Sox17 CGCACGGAATTTGAACAGTA GGATCAGGGACCTGTCACAC GSC GAGGAGAAAGTGGAGGTCTGGTT CTCTGATGAGGACCGCTTCTG Immature human liver organoids markers CYP3A7 GAAACACAGATCCCCCTGAA TCAGGCTCCACTTACGGTCT Mature human liver organoids markers HNF4α CATGGCCAAGATTGACAACCT TTCCCATATGTTCCTGCATCAG ALB TTG GCA CAA TGA AGT GGG TA AAA GGC AAT CAA CAC CAA GG A1AT CCACCGCCATCTTCTTCCTGCCTGA GAGCTTCAGGGGTGCCTCCTCTGTG CYP3A4 TGTGCCTGAGAACACCAGAG GTGGTGGAAATAGTCCCGTG GADPDH CCATCTTCCAGGAGCGAG GCAGGAGGCATTGCTGAT
Solutions
Vitronectin (see Recipes)
0.5 mM ultrapure EDTA (see Recipes)
Activin A (see recipe)
Bone morphogenetic protein 4 (see Recipes)
Fibroblast growth factor 4 (see Recipes)
0.1% (m/v) bovine serum albumin (see Recipes)
CHIR99021 (see Recipes)
Retinoic acid (see Recipes)
Hepatocyte growth factor (see Recipes)
Dexamethasone (see Recipes)
Oncostatin M (see Recipes)
Fibroblast growth factor 10 (see Recipes)
Epidermal growth factor (see Recipes)
A83-01 (see Recipes)
Rho kinase protein (ROCK) inhibitor (Y-27632) (see Recipes)
Cell culture media
hiPSCs maintenance media: complete essential 8 medium (see Recipes)
Definitive endoderm induction (days 1–3) media: Complete RPMI medium (see Recipes)
Foregut spheroid (FGS) induction (days 4–6) media: Complete Advanced DMEM/F2 (see Recipes)
Human liver organoids (HLO) formation (days 7–10) media 1: Advanced DMEM/F12 + retinoic acid (see Recipes)
Hepatocyte maturation (days 11–25) media: Hepatocyte culture medium (HCM) (see Recipes)
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
First strand CDNA synthesis cocktail 1 (see Recipes)
cDNA synthesis master mix 2 (see Recipes)
qRT-PCR reagents mix (see Recipes)
Recipes
Vitronectin solution
Reagents Final concentration Amount Vitronectin 9 µg/mL 0.9 mg/mL DPBS 1× 990 mL Total n/a 1,000 mL 0.5 mM ultrapure EDTA
Reagents Final concentration Amount Ethylenediaminetetraacetic acid 0.5 mM 10 mL DPBS 1× 9,990 mL Total n/a 10,000 mL Store at room temperature (RT) and use on the day of preparation.
Activin A solution
Reagents Final concentration Amount Activin A 100 µg/mL 10 µg HCl 4 mM 100 mL Total n/a 100 mL Prepare 1,000 ng/mL working solution in RPMI-1640 + GlutaMAX and store at -20 °C until needed for the experiment.
Bone morphogenetic protein 4 (BMP-4) solution
Reagents Final concentration Amount BMP-4 100 µg/mL 10 µg 0.1% BSA in 4 mM HCl n/a 100 mL Total n/a 100 mL Prepare 1,000 ng/mL working solution in RPMI-1640 + GlutaMAX and store at -20 °C until needed for experiment.
Fibroblast growth factor 4 (FGF-4) solution
Reagents Final concentration Amount FGF-4 100 µg/mL 25 µg 0.1% BSA in PBS (Recipe 6) n/a 100 mL Total n/a 100 mL Prepare 1,000 ng/mL working solution in Advanced DMEM/F12 and store at -20 °C until needed for the experiment.
0.1% (m/v) bovine serum albumin (BSA) in PBS
Reagents Final concentration Amount Bovine serum albumin (BSA) 0.1% (m/v) 0.1 g H2O n/a 100 mL Total n/a 100 mL CHIR99021 solution
Reagents Final concentration Amount CHIR99021 5 mg/mL 5 mg DMSO n/a 1,000 mL Total n/a 1,000 mL Prepare 1 mM working solution in advanced DMEM/F12 and store at -20 °C until needed for experiments.
Retinoic acid (RA) solution
Reagents Final concentration Amount Retinoic acid 50 mg/mL 50 mg DMSO n/a 1,000 mL Total n/a 1,000 mL Prepare 1 mM working solution in advanced DMEM/F12 and store at -20 °C until needed for experiments.
Hepatocyte growth factor solution
Reagents Final concentration Amount Hepatocyte growth factor (HGF) 100 mg/mL 5 mg 0.1% BSA in PBS (Recipe 6) n/a 50 mL Total n/a 50 mL Prepare 1,000 ng/mL working solution in hepatocyte culture medium (HCM) and store at -20 °C until needed for the experiment.
Dexamethasone (Dex) solution
Reagents Final concentration Amount Dexamethasone (Dex) 10 mM 5 mg DMSO n/a 1,000 mL Total n/a 1,000 mL Prepare 1,000 µM working solution in HCM and store at -20 °C until needed for the experiment.
Oncostatin M (OSM) solution
Reagents Final concentration Amount Oncostatin M (OSM) 100 mg/mL 10 µg 0.1% BSA in PBS (Recipe 6) n/a 100 mL Total n/a 100 mL Prepare 1,000 ng/mL working solution in HCM and store at -20 °C until needed for the experiment.
Fibroblast growth factor 10 (FGF-10) solution
Reagents Final concentration Amount Fibroblast growth factor 10 (FGF-10) 250 mg/mL 25 µg 0.1% BSA in PBS (Recipe 6) n/a 100 mL Total n/a 100 mL Prepare 1,000 ng/mL working solution in advanced DMEM/F12 and store at -20 °C until needed for experiments.
Epidermal growth factor (EGF) solution
Reagents Final concentration Amount Epidermal growth factor 500 mg/mL 200 µg/mL 0.1% (m/v) BSA in PBS n/a 400 mL Total n/a 400 mL Prepare 1,000 ng/mL working solution in advanced DMEM/F12 and store at -20 °C until needed for experiments.
A 83-01 solution
Reagents Final concentration Amounts A 83-01 10 mg/mL 10 mg DMSO n/a 1,000 mL Total n/a 1,000 mL Prepare 1 mM working solution in advanced DMEM/F12 and store at -20 °C until needed for experiments.
Rho kinase protein (ROCK) inhibitor (Y-27632) solution
Reagents Final concentration Amount Y-27632 10 mM 5 mg DMSO n/a 1,000 mL Total n/a 1,000 mL Store at -20 °C for up to six months.
Cell culture media
hiPSCs maintenance media: complete essential 8 medium
Reagents Final concentration Amount E8M 1× 485 mL E8 supplement 1× 10 mL P/S 1× 5 mL Total n/a 500 mL Alternatives: other hiPSC culture media can be used such as mTeSR1, StemFlex, and TeSR-E8. Swirl the bottle to mix contents properly, label with name and date prepared, filter, and store at 4 °C for up to 14 days. Alternatively, 50 mL aliquots can be made and stored at -20 °C.
Definitive endoderm induction (days 1–3) media: Complete RPMI medium
Regents Final concentration Amount RPMI-1640 1× 482 mL P/S 1× 5 mL HEPES 25 mM 12.5 mL Activin A 100 ng/mL - BMP-4 50 ng/mL - KSR 1× - Total n/a 500 mL Amount of Activin A, BMP4, and KSR are not shown on the Table as we prepare them fresh every day and they are dependent on the number of wells and volumes you are working with.
Swirl the bottle to mix contents thoroughly, label with name and date prepared, filter, and store at 4 °C for up to 14 days. Alternatively, 50 mL aliquots can be made and stored at -20 °C. Day 1: RPMI media supplemented with 100 ng/mL Activin A and 50 ng/mL BMP-4. Day 2: RPMI media supplemented with 100 ng/mL Activin A and 0.2% KSR. Day 3: RPMI media supplemented with 100 ng/mL Activin A and 2% KSR without BMP4 (see Notes 1–3).
Foregut spheroid (FGS) induction (days 4–6) media: Complete Advanced DMEM/F2
Reagents Final concentration Amount Advanced DMEM/F12 1× 475 mL B27 supplement 1× 5 mL N2 supplement 1× 10 mL GlutaMAX 1× 5 mL P/S 1× 5 mL FGF-4 - - CHIR99021 - - Total n/a 500 mL Amounts of FGF-4 and CHIR99021 are not shown on the Table as we prepare them fresh every day and they are dependent on the number of wells and volumes you are working with. Swirl the bottle to mix contents thoroughly, label with name and date prepared, filter, and store at 4 °C for up to 14 days. Alternatively, 50 mL aliquots can be made and stored at -20 °C. Days 4–6 media: advanced DMEM/F12 supplemented with 3 µM CHIR99021 and 500 ng/mL FGF-4 (see Notes 1–3).
Human liver organoids (HLO) formation (days 7–10) media 1: Advanced DMEM/F12 + retinoic acid
Reagents Final concentration Amount Advanced DMEM/F12 1× 475 mL B27 supplement 1× 5 mL N2 supplement 1× 10 mL GlutaMAX 1× 5 mL P/S 1× 5 mL Retinoic acid 2 µM 1 mL Total n/a 500 mL Place the media in the dark or cover with foil.
Hepatocyte maturation (days 11–25) media: Hepatocyte culture medium (HCM)
Reagent Final concentration Amount HCM basal medium 1× 500 mL HGF - - OSM - - Dex - - Total n/a 500 mL Amounts for HGF, OSM, and Dex are not shown on the Table as we prepare them fresh every day in small quantities depending on the number of wells and volumes you are working with. Swirl the bottle to mix contents thoroughly, label with name and date prepared, filter, and store at 4 °C for up to 14 days. Alternatively, 50 mL aliquots can be made and stored at -20 °C. Days 11–25 media: supplement HCM medium with 10 ng/mL HGF, 0.1 µM Dex, and 20 ng/mL OSM.
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
First-strand CDNA synthesis cocktail 1
Reagents Final concentration Amount mRNA 2 µg - Oligo dT primer n/a 1 µL ddH2O n/a - Total n/a 9 µL Note: The amount of mRNA will depend on the concentration of RNA per sample; the volume of water will be calculated based on the concentration value of RNA.
cDNA synthesis master mix 2
Reagents Amount 5 first synthesis strand buffer 5 µL dNTPs mix 1 µL RNase inhibitor 1 µL MgCl2 2 µL ImPromp II reverse transcriptase 1 µL ddH2O 6 µL Total 16 µL qRT-PCR reagents mix
Reagents Final concentration Amount SYBR Green PCR Master Mix n/a 6.25 µL Forward primer (10 µM) 10 µM 0.5 µL Reverse primer (10 µM) 10 µM 0.5 µL ddH2O n/a 4.25 µL cDNA n/a 1 µL Total n/a 12.5 µL
Laboratory supplies
Nunclon Sphera multi-well plates (Thermo Fisher Scientific, catalog number: 174930)
Sterile 15 mL Falcon tubes (Nest Biotechnology, catalog number: 602072)
Sterile 50 mL Falcon tubes (Nest Biotechnology, catalog number: 601001)
Equipment
Flow Cytometer (BD Biosciences, model: BD LSR Fortessa)
Biosafety cabinet suitable for cell culture (Nuaire)
Centrifuge (United Scientific, model: Orto Alresa)
Microplate Luminometer (Promega, model: GLOMAX 96)
Heat block (Acorn Scientific, model: Stuart)
Spectrophotometer (Thermo Fisher Scientific, model: NanoDropTM 2000)
Phase contrast/inverted microscope (Leica Microsystems, model: Leica DM IL LED)
Thermal Cycler (Thermo Fisher Scientific, model: QuantStudio 3 RT-PCR Systems)
Shellab CO2 Water Jacket HEPA Filter Incubator (United Scientific, model: SC05W-2)
Vortex machine (Labnet International)
Software and datasets
GraphPad Prism (GraphPad Software Inc.; version 5)
FlowJo software (Treestar; version 10)
Procedure
文章信息
稿件历史记录
提交日期: Dec 5, 2023
接收日期: Apr 30, 2024
在线发布日期: Jul 18, 2024
出版日期: Aug 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Maepa, S. W., Marakalala, M. J. and Ndlovu, H. (2024). Generation of Multicellular 3D Liver Organoids From Induced Pluripotent Stem Cells as a Tool for Modelling Liver Diseases. Bio-protocol 14(15): e5042. DOI: 10.21769/BioProtoc.5042.
分类
干细胞 > 多能干细胞 > 再生医学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link