- EN - English
- CN - 中文
Microscale Thermophoresis (MST) as a Tool to Study Binding Interactions of Oxygen-Sensitive Biohybrids
利用微尺度热泳技术研究氧敏感生物杂合体的结合相互作用
(*contributed equally to this work) 发布: 2024年08月05日第14卷第15期 DOI: 10.21769/BioProtoc.5041 浏览次数: 2737
评审: Joana Alexandra Costa ReisPhilipp A.M. SchmidpeterDjamel Eddine Chafai
Abstract
Microscale thermophoresis (MST) is a technique used to measure the strength of molecular interactions. MST is a thermophoretic-based technique that monitors the change in fluorescence associated with the movement of fluorescent-labeled molecules in response to a temperature gradient triggered by an IR LASER. MST has advantages over other approaches for examining molecular interactions, such as isothermal titration calorimetry, nuclear magnetic resonance, biolayer interferometry, and surface plasmon resonance, requiring a small sample size that does not need to be immobilized and a high-sensitivity fluorescence detection. In addition, since the approach involves the loading of samples into capillaries that can be easily sealed, it can be adapted to analyze oxygen-sensitive samples. In this Bio-protocol, we describe the troubleshooting and optimization we have done to enable the use of MST to examine protein–protein interactions, protein–ligand interactions, and protein–nanocrystal interactions. The salient elements in the developed procedures include 1) loading and sealing capabilities in an anaerobic chamber for analysis using a NanoTemper MST located on the benchtop in air, 2) identification of the optimal reducing agents compatible with data acquisition with effective protection against trace oxygen, and 3) the optimization of data acquisition and analysis procedures. The procedures lay the groundwork to define the determinants of molecular interactions in these technically demanding systems.
Key features
• Established procedures for loading and sealing tubes in an anaerobic chamber for subsequent analysis.
• Sodium dithionite (NaDT) could easily be substituted with one electron-reduced 1,1'-bis(3-sulfonatopropyl)-4,4'-bipyridinium [(SPr)2V•] to perform sensitive biophysical assays on oxygen-sensitive proteins like the MoFe protein.
• Established MST as an experimental tool to quantify binding affinities in novel enzyme–quantum dot biohybrid complexes that are extremely oxygen-sensitive.
Keywords: Microscale thermophoresis (微尺度热泳)Graphical overview
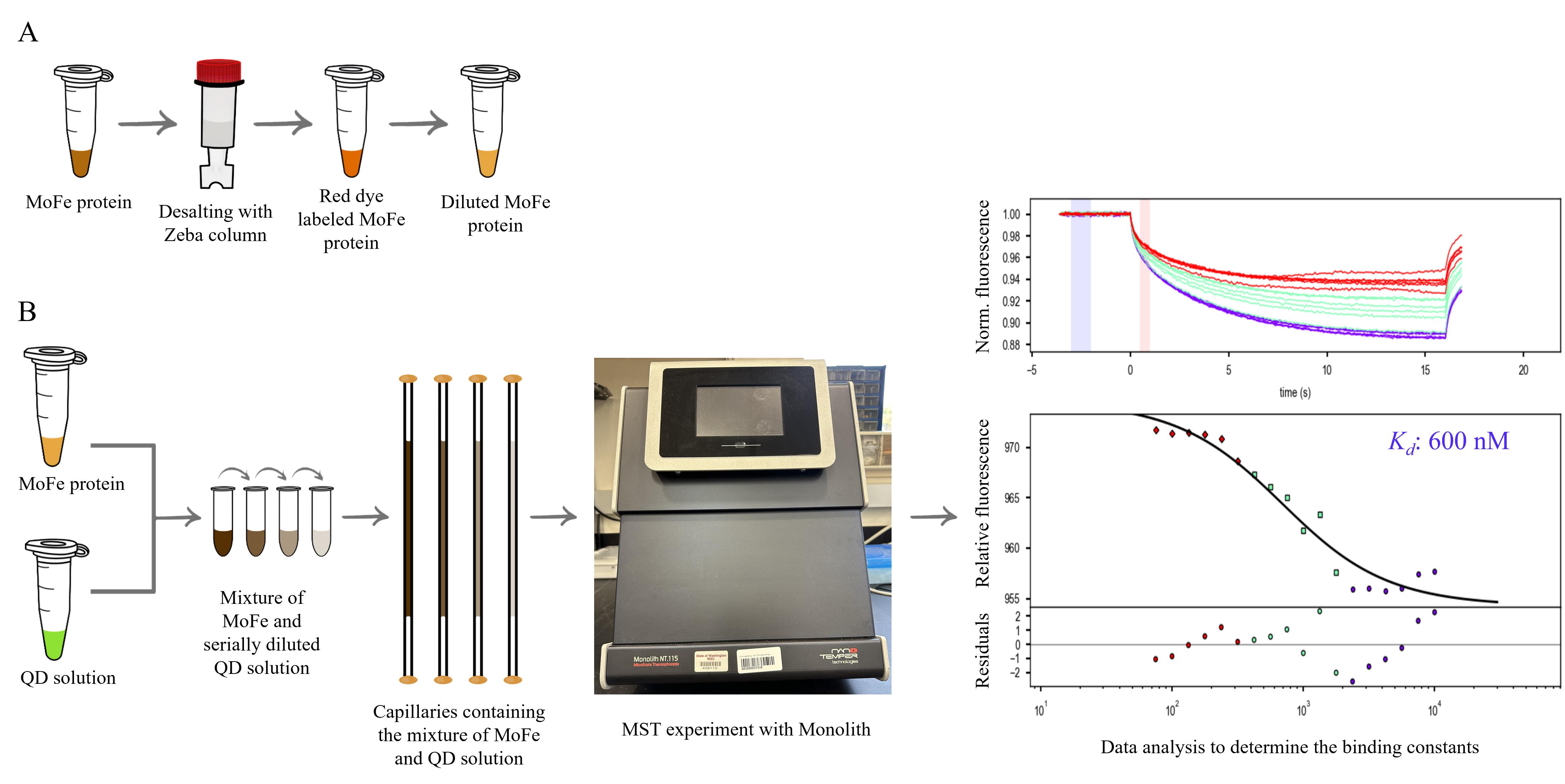
Flowchart of the experimental workflow to determine the dissociation constant (Kd) between the MoFe protein and quantum dots (QDs). A) Preparation of the MoFe protein for the microscale thermophoresis (MST) experiment: The MoFe protein, which was originally purified in HEPES buffer, was buffer exchanged with MOPS-(SPr)2V• buffer using a Zeba column, diluted, and stored in liquid nitrogen. The MoFe protein in MOPS-(SPr)2V• buffer is diluted in MOPS-(SPr)2V•-Tween buffer and labeled with RED-tris-NTA second-generation dye to achieve the concentration required for the MST experiments. B) Experimental flowchart of the MST experiment: The Red dye–labeled MoFe protein and unlabeled QD solution stocks are prepared separately. Prepare a serial dilution of QD solution and mix them with an equal concentration of Red dye–labeled MoFe protein. Transfer the MoFe protein–QD mixture into capillary tubes individually and seal them with wax. Transfer the capillaries into Monolith [1] and perform the MST experiment, which is followed by data analysis and determination of the dissociation constant (Kd) between the MoFe protein and QDs. The top panel of the graph represents the changes observed in the fluorescence intensity during the MST experiment. The bottom panel is the graph depicting the calculated value of the Kd based on the observed fluorescence changes (Y-axis) as a function of the concentration of the interacting QD (X-axis). The lower part of the bottom panel represents the residuals, depicting the quality of the experimental fit.
Background
Quantification of biomolecular interactions is essential as they are critical to drug design, pharmaceutical, and bioprocessing industries. There are several techniques used to determine the strength of molecular interactions including isothermal titration calorimetry (ITC), surface plasmon resonance (SPR), nuclear magnetic resonance (NMR), and biolayer interferometry (BLI). Although these techniques have wide applications, there are several challenges associated with them in studying oxygen-sensitive samples. Microscale thermophoresis (MST), on the other hand, is a highly sensitive method where the intermolecular interactions are studied based on changes in fluorescence intensity of the target molecule as a function of the temperature-based difference in movement between bound and unbound states across a thermal gradient [1,2]. This Bio-protocol focuses on adapting the technique to analyze molecular interactions for highly oxygen-sensitive metalloenzymes and nanocrystalline quantum dots (QDs). We found that it was suboptimal to operate the NanoTemper MST within an anaerobic chamber, necessitating the development of a procedure to load and seal samples in an anaerobic chamber for analysis on the bench in the air. We achieved this by preparing the samples and sealing the MST capillaries inside the mBraun glove box, using capillary wax. Although this proved to be a successful strategy, there still was a need to protect extremely oxygen-sensitive samples like the MoFe protein and the Fe protein of nitrogenase from trace amounts of oxygen that might be introduced during sample preparation. Strong reducing agents like sodium dithionite (NaDT) are routinely used for this purpose; however, NaDT quenches the intensity of the fluorescence signal at even low concentrations. To overcome this, we have employed a novel, one electron-reduced viologen-based 1,1'-bis(3-sulfonatopropyl)-4,4'-bipyridinium radical anion [(SPr)2V•] that does not quench the fluorescence signal, instead of NaDT as a reducing agent in our experiments [3–5]. To check if the (SPr)2V• is a favorable and viable option, we have tested it for studying MoFe protein and Fe protein interactions. These proteins were selected because they are known to be extremely oxygen-sensitive; if our protocol with (SPr)2V• and sealing of the capillaries inside the mBraun works, this can be adapted as a reliable system to study interactions between any molecules that are oxygen-sensitive. After successfully validating our results, we have further developed MST protocols to study interactions of MoFe protein–cadmium sulfide (CdS) nanocrystal biohybrids. Specifically, we adapted the approach to examine CdS QDs of various sizes, which have been shown to interact with several metalloenzymes where the photoexcited conduction band electrons from the QDs could be transferred to the active site of the enzyme, triggering catalysis [6–9]. This emerging class of novel enzyme-QD biohybrids is of utmost significance as it could redefine bio-catalysis [10]. We have successfully demonstrated light-driven reduction of N2 to NH3 using novel MoFe-CdS nanocrystal biohybrids [11,12]. Although the reactivity of MoFe protein and CdS QD mixtures have been studied, there has been no characterization of the molecular interactions prior to our work [13]. We have now successfully developed the protocols for determining the dissociation constants (Kd) of these novel biohybrids involving MoFe protein and the CdS QDs. Here, we have articulated a detailed protocol for determining the Kd between the MoFe protein and 3.7 nm CdS QDs, which was reported in Pellows et al. [13].
Materials and reagents
Reagents
His-Tag labeling Kit RED-tris-NTA 2nd Generation (NanoTemper, catalog number: MO-L018)
Pierce BCA Protein Assay kit (Thermo Fisher, catalog number: 23225)
Capillary wax (Hampton Research, catalog number: HR4-328)
Liquid nitrogen
Double-distilled Milli-Q water
Sodium chloride (NaCl) (Sigma, catalog number: S9888-5KG)
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Sigma, catalog number: H3375-25G)
3-(N-morpholino) propanesulfonic acid (MOPS) (Sigma, catalog number: M1254-25G)
Sodium dithionite (Fisher Scientific, catalog number: 10274490)
Tween-20 (Sigma, catalog number: P1379-25ML)
One electron-reduced 1,1'-bis(3-sulfonatopropyl)-4,4'-bipyridinium [(SPr)2V•] (details in Procedure)
MoFe protein (details in Procedure)
3.7 nm CdS QD (details in Procedure)
Solutions
HEPES buffer (see Recipes)
MOPS-(SPr)2V• buffer (see Recipes)
MOPS-(SPr)2V•-Tween buffer (see Recipes)
QD buffer (see Recipes)
Recipes
HEPES buffer
Reagent Final concentration Amount HEPES 50 mM, pH 7 119 mg NaCl 150 mM 87.6 mg Sodium dithionite (1 M) 2 mM 20 μL H2O n/a 9 mL Make the final volume to 10 mL MOPS-(SPr)2V• buffer
Reagent Final concentration Amount MOPS 50 mM, pH 7 104 mg NaCl 150 mM 87.6 mg (SPr)2V• 50 mM 1 mM 0.2 mL H2O n/a 9 mL Make the final volume to 10 mL MOPS-(SPr)2V•-Tween buffer
Reagent Final concentration Amount MOPS 50 mM, pH 7 104 mg NaCl 10 mM 5.84 mg (SPr)2V• 50 mM 50 μM 10 μL Tween-20 (100%) 0.05% 5 μL H2O n/a 9 mL Make the final volume to 10 mL QD buffer
Reagent Final concentration Amount MOPS 50 mM, pH 7 104 mg (SPr)2V• 50 mM 50 μM 10 μL H2O n/a 9 mL Make the final volume to 10 mL Note: All buffers must be thoroughly degassed before moving them inside the mBraun glove box. This experiment requires only small amounts of buffer, and (SPr)2V• in buffers can degrade upon long-term storage.
Laboratory supplies
Monolith capillaries (NanoTemper, catalog number: MO-K022)
1.5 mL low protein binding microcentrifuge tubes (Thermo Fisher, catalog number: 90410)
0.2 mL thin-wall low protein binding microcentrifuge tubes (BIOplastics, catalog number: K77301)
0.5 mL low protein binding microcentrifuge tubes (Sarstedt Inc, catalog number: 72.704.600)
0.5 mL Zeba spin desalting columns, 7K MWCO (Thermo Fisher, catalog number: 89882)
50 mL tubes (VWR, catalog number: 89039-658)
50 mL tubes (VWR, catalog number: 89039-666)
10 μL pipette tips (VWR, catalog number: 470146-272)
200 μL pipette tips (VWR, catalog number: 470146-222)
1,000 μL pipette tips (VWR, catalog number: 470146-264)
Fisher brand 96-well PCR tube racks (Fisher Scientific, catalog number: 03-448-20)
VWR 80-well microtube racks (VWR, catalog number: 82024-469)
10 mL Wheaton crimp vials (Sigma, catalog number: DWK223686)
Aluminum crimp seals (VWR, catalog number: 97047-818)
Rubber stoppers (Grainger, catalog number: W224100-202)
500 µL gastight syringe (Hamilton, catalog number: 81265)
20 mL sample vials (Grainger, catalog number: 986710)
Equipment
Nanodrop (Thermo Scientific, NanoDrop One, catalog number: ND-ONEC-W)
Microscale thermophoresis (NanoTemper, model: Monolith NT.115)
Dry bath (VWR, Standard Dry Block Heater, catalog number: 75838-286)
Mini centrifuge (Eppendorf, MiniSpin, catalog number: 022620100)
Glove box (mBraun, model: UNIlab pro SP)
Vortexing machine (VWR, Analog Vortex Mixer, catalog number: 10153-838)
Software and datasets
MO.Control (NanoTemper, version: 1.6.1)
PALMIST software (The University of Texas Southwestern Medical Center, version 1.5.8)
Procedure
文章信息
稿件历史记录
提交日期: Mar 27, 2024
接收日期: Jun 24, 2024
在线发布日期: Jul 18, 2024
出版日期: Aug 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Jagilinki, B. P., Willis, M. A., Mus, F., Sharma, R., Pellows, L. M., Mulder, D. W., Yang, Z. Y., Seefeldt, L. C., King, P. W., Dukovic, G. and Peters, J. W. (2024). Microscale Thermophoresis (MST) as a Tool to Study Binding Interactions of Oxygen-Sensitive Biohybrids. Bio-protocol 14(15): e5041. DOI: 10.21769/BioProtoc.5041.
分类
生物物理学 > 生物工程 > 纳米材料
生物化学 > 蛋白质 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link