- EN - English
- CN - 中文
An Experimental Protocol for the Boyden Chamber Invasion Assay With Absorbance Readout
Boyden小室法侵袭实验的吸光度检测实验方案
发布: 2024年08月05日第14卷第15期 DOI: 10.21769/BioProtoc.5040 浏览次数: 2755
评审: Valérian DORMOYChhuttan L MeenaSalah Boudjadi
Abstract
The phenomenon of cell invasion is an essential step in angiogenesis, embryonic development, immune responses, and cancer metastasis. In the course of cancer progression, the ability of neoplastic cells to degrade the basement membrane and penetrate neighboring tissue (or blood vessels and lymph nodes) is an early event of the metastatic cascade. The Boyden chamber assay is one of the most prevalent methods implemented to measure the pro- or anti-invasive effects of drugs, investigate signaling pathways that modulate cell invasion, and characterize the role of extracellular matrix proteins in metastasis. However, the traditional protocol of the Boyden chamber assay has some technical challenges and limitations. One such challenge is that the endpoint of the assay involves photographing and counting stained cells (in multiple fields) on porous filters. This process is very arduous, requires multiple observers, and is very time-consuming. Our improved protocol for the Boyden chamber assay involves lysis of the dye-stained cells and reading the absorbance using an ELISA reader to mitigate this challenge. We believe that our improved Boyden chamber methodology offers a standardized, high-throughput format to evaluate the efficacy of various drugs and test compounds in influencing cellular invasion in normal and diseased states. We believe that our protocol will be useful for researchers working in the fields of immunology, vascular biology, drug discovery, cancer biology, and developmental biology.
Key features
• Measurement of tumor invasion using human cancer cells.
• Ability to measure the pro-invasive/anti-invasive activity of small molecules and biological modifiers.
• Measurement of chemotaxis, chemokines, trafficking of immune cells, and proteolytic activity of matrix metalloproteinases, lysosomal hydrolysates, collagenases, and plasminogen activators in physiological and pathological conditions.
• Investigation of the role of extracellular matrix proteins in the crosstalk between endothelial, epithelial, muscle, or neuronal cells and their adjacent stroma.
Keywords: Invasion (侵袭)Graphical overview
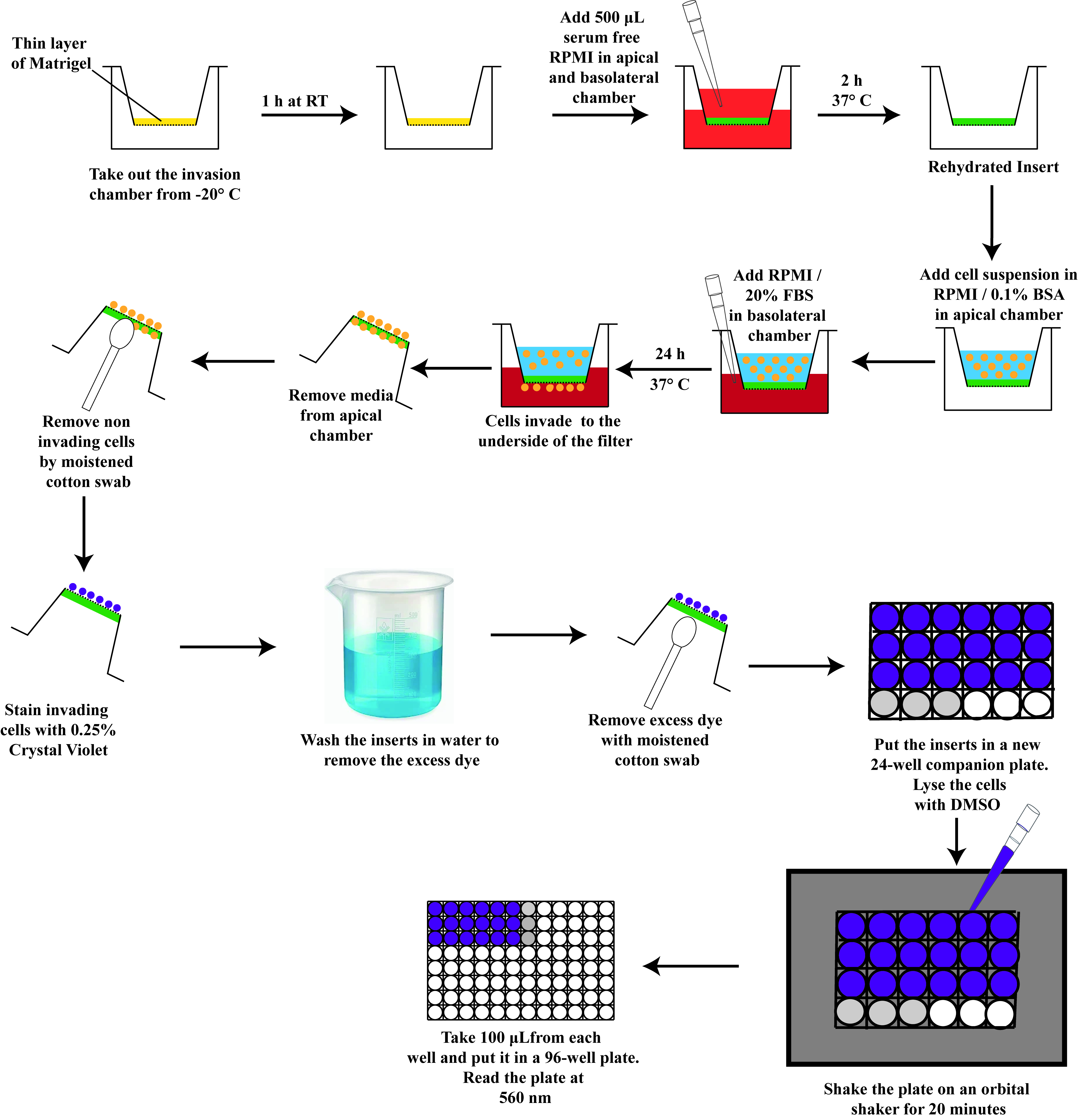
A schematic diagram depicting the steps of the Boyden chamber assay
Background
The process of metastasis is responsible for the majority of deaths in cancer patients [1]. Metastasis is a complex multi-step process comprised of primary tumor cell invasion into the surrounding tissue and extravasation into and intravasation out of the blood or lymphatic system, resulting in tumor cell colonization at distant sites and organs in the body [2,3]. Out of these processes, the process of tumor invasion has been the foundation of most drug-discovery studies. Invasion refers to the process by which cancer cells penetrate the basement membrane and launch themselves into circulation in the adjacent blood vessels or lymph nodes [4,5]. The Boyden chamber assay is the most prevalent method to measure this invasion capacity [6,7]. This assay requires two compartments separated by a microporous membrane coated with a Matrigel basement membrane matrix. In most versions of the Boyden chamber assay, cancer cells are placed in the upper compartment, and the lower compartment contains a robust chemoattractant. Under the influence of the chemoattractant, the cancer cells secrete proteases to degrade the Matrigel basement membrane, then migrate through the pores of the membrane into the lower compartment. A challenge to the traditional Boyden chamber assay is the arduous and time-consuming procedures of fixation and staining of the cells on the bottom surface of the porous membrane and subsequent photography and manual cell counting [6]. Our improved method of the Boyden chamber assay allows the results of the assay to be measured on a microplate reader. Therefore, our Boyden chamber assay protocol may be adapted to a high-throughput format. Currently, many research efforts are directed at improving our understanding of the metastatic cascade to advance our ability to design anti-cancer drugs, which will specifically target the process of metastasis. Out of all the events of metastasis, the process of tumor invasion has been the primary focus of drug discovery efforts [8,9]. The overarching goal of our improved Boyden chamber assay is to provide a simple, robust, and reproducible cell-based assay with a large-scale capability of evaluating and measuring the invasive ability of various cancer cells. We believe that our improved protocol of the Boyden chamber assay may pave the way for more rapid identification of novel anti-cancer drugs to combat metastasis.
Materials and reagents
Biological materials
DMS114 human small cell lung cancer (SCLC) cells [American Type Culture Collection (ATCC), catalog number: CRL-2066]. The cells should be grown to 70%–80% confluence in DMS114 culture media (see Recipe 3) and kept in a humidified environment at 37 °C (in the presence of 5% CO2) in a cell culture incubator [10]. Although we are describing the BCA protocol using DMS114 human SCLC cells, the pro-invasive activity of any relevant cancer cell line may be measured using this assay. Normal cells display pro-invasive activity under specific biological conditions. Such conditions may include treatment with growth factors, steroids, and carcinogenic chemicals. The BCA may be used to measure the effect of such chemicals on the invasion of normal cells.
Reagents
CorningTM BioCoatTM MatrigelTM invasion chamber inserts coated with Matrigel matrix. These invasion chambers are sold in two formats. The first is a 6-well format (Fisher Scientific, catalog number: 08-774-121). The second is a 24-well format (Fisher Scientific, catalog number: 08-774-122). Store at -20 °C. We use the 24-well format for the protocol described in the present manuscript
Sterile bovine serum albumin (BSA) solution at a concentration of 30% (w/v) in DPBS (Millipore-Sigma, catalog number: A7596)
Roswell Park Memorial Institute (RPMI)-1640 medium (ATCC, catalog number: 30-2001). Store at 4 °C, 1 M HEPES (Thermo Fisher, catalog number: 15630080)
Fetal bovine serum (FBS) (ATCC, catalog number: 30-2020). Long-term storage for FBS is at -70 °C. In our laboratory, FBS is aliquoted into 50 mL centrifuge tubes and stored at -20 °C. The FBS aliquot is thawed and stored at 4 °C for immediate use
Trypsin-EDTA (0.25% Trypsin, 0.53 mM EDTA) (ATCC, catalog number: 30-2101). Long-term storage for Trypsin-EDTA is at -20 °C. However, Trypsin-EDTA is aliquoted and stored at 4 °C for immediate use
1 M HEPES (Thermo Fisher Scientific, catalog number 15630080). Long-term storage for 1 M HEPES is at -20 °C. However, HEPES is aliquoted and stored at 4 °C for immediate use
Penicillin-streptomycin solution (ATCC, catalog number: 30-2300)
Dulbecco’s phosphate buffered saline (PBS) without calcium and magnesium (Corning, Fisher, catalog number: 21-031-CM). Store at 4 °C
Corning cell counting chamber (Fisher Scientific, catalog number: 07-200-988)
Capsaicin (Sigma-Aldrich, catalog number: M2028-50MG). Capsaicin is the hot and spicy ingredient of chili peppers. It is a potent agonist of transient receptor potential cation channel subfamily V member 1 (TRPV1). Store at 4 °C
PP2 is a potent and selective inhibitor of the Src family tyrosine kinases [11]. PP2 was manufactured by Enzo Biosciences (Enzo Biosciences, catalog number: 50-201-0680) and obtained from Thermo Fisher Scientific. The PP2 is delivered as powder and stored at -20 °C. The PP2 is dissolved in DMSO (Corning DMSO, Fisher, catalog number: MT-25950CQC) at a final concentration of 10 mM. The PP2 solution is aliquoted into microfuge tubes and stored at -20 °C
Dimethyl sulfoxide (DMSO), ACS certified (Fisher BioReagents, catalog number: D128-500). Store at room temperature
Aqua Solutions crystal violet 1% w/v aqueous (Fisher Scientific, catalog number: NC9002731). Store at room temperature
Solutions
Stock capsaicin (100 mM) (see Recipes)
WS-CAP-100X (2 mM) (see Recipes)
VEH-CAP-100X (see Recipes)
Stock PP2 (10 mM) (see Recipes)
WS-PP2-100X (500 µM) (see Recipes)
VEH-PP2 (see Recipes)
Crystal violet staining solution (see Recipes)
DMS114 culture medium (see Recipes)
RPMI-BSA (see Recipes)
Recipes
| Reagent | Final concentration | Solvent | Volume of each aliquot | Total volume | Storage |
|---|---|---|---|---|---|
| Stock capsaicin | 100 mM | DMSO | 25 µL | 100 µL | -20 °C |
| WS-CAP-100X | 2 mM | RPMI/0.1% BSA | Made fresh before use | 100 µL | On ice until use |
| VEH-CAP-100X | 2% DMSO (v/v) | Serum-free RPMI | NA | 100 µL | Made fresh before use |
| Stock PP2 | 10 mM | DMSO | 25 µL | 333 µL | -20 °C |
| WS-PP2-100X | 500 µM | RPMI/0.1% BSA | Made fresh before use | 100 µL | Made fresh before use |
| VEH-PP2-100X | 5% DMSO (v/v) | Serum-free RPMI | NA | 100 µL | On ice until use |
| Crystal violet staining solution | 0.25% crystal violet | Milli Q Water | NA | 20 mL | Room temperature |
Stock capsaicin (100 mM), 1 mL
Weigh 30.5 mg of capsaicin in a sterile autoclaved amber-colored 1.5 mL microfuge tube. In a laminar flow hood, add 1 mL of DMSO to the tube containing capsaicin. Vortex to mix. Aliquot in sterile amber-colored 1.5 mL microfuge tubes. We typically put 25 µL of capsaicin stock solution in each aliquot. Store the aliquots at -20 °C. The aliquots are stable for one month at -20 °C. Laboratory personnel should wear gloves, mask, and face goggles when working with powdered capsaicin. Capsaicin can cause intense irritation if it comes in contact with the skin, face, or eyes.
WS-CAP-100X (2 mM), 100 µL
Take out an aliquot of 100 mM capsaicin solution from -20 °C. Put the tube in a 37 °C water bath for 5 min (until the entire solution is thawed). Vortex briefly to mix. Add 2 µL of 100 mM stock capsaicin to 98 µL of RPMI-BSA (in an autoclaved amber-colored 1.5 mL microfuge tube) in a laminar flow hood. Vortex to mix. Keep the solution on ice until used in the experiment. We make a fresh solution of WS-CAP before every assay and discard any leftover solution.
VEH-CAP-100X, 100 µL
Add 2 µL of DMSO to 98 µL of RPMI-BSA (in an autoclaved amber-colored 1.5 mL microfuge tube) in a laminar flow hood. Vortex to mix. We do not store the VEH-CAP-100X solution. We make a fresh solution of VEH-CAP before every assay and discard any leftover solution.
Stock PP2 (10 mM), 333 µL
Add 333 µL of DMSO to the bottle of 1 mg of PP2 in a laminar flow hood. Vortex to mix. Aliquot in autoclaved amber-colored 1.5 mL microfuge tubes (we put 25 µL of PP2 solution in each aliquot). Store the aliquots in -20 °C. The aliquots are stable for one month at -20 °C.
WS-PP2-100X (500 µM), 1 mL
Take out an aliquot of 10 mM PP2 solution from -20 °C. Put the tube in a 37 °C water bath for 5 min (until the entire solution is thawed). Vortex briefly to mix. Add 5 µL of stock solution of DMSO to 95 µL of RPMI-BSA (in an autoclaved amber-colored 1.5 mL microfuge tube) in a laminar flow hood. Vortex to mix. Keep the solution on ice until it is used for the experiment. We make a fresh solution of WS-PP2 before every assay and throw away the leftover solution.
VEH-PP2, 1 mL
Add 5 µL of DMSO to 95 µL of RPMI-BSA (in an autoclaved amber-colored 1.5 mL microfuge tube) laminar flow hood. Vortex to mix. We make a fresh solution of vehicle before every assay and do not store it.
Crystal violet staining solution, 20 mL
Add 5 mL of 1% crystal violet solution to 15 mL of Milli Q water. Vortex to mix. Store the solution at room temperature.
DMS114 culture medium
DMS114 cells were cultured following the published protocols [10]. All procedures are performed in a laminar flow hood. The DMS114 culture medium consists of RPMI-1640 with 1% Penicillin streptomycin solution (containing 100 units/mL penicillin, 50 µg/mL streptomycin), 25 mM HEPES, and 10% FBS. ATCC RPMI-1640 (30-2001 formulation) comes formulated with 10 mM HEPES. We then add an additional 7.5 mL of 1 M HEPES to 500 mL of RPMI-1640 to obtain a 25 mM concentration of HEPES. Store the media at 4 °C. To note, many laboratories add 50 mL of FBS to the full bottle of media to obtain 10% (v/v). We do not typically add FBS to the entire RPMI bottle. The rationale for this is to facilitate the most efficient use of each RPMI bottle. Many of our experiments require serum-free media, so we add the FBS as needed. The cells should be grown to 70%–80% confluence using the media described above and kept in a sterile cell culture incubator with a humidified environment, set at 37 °C with 5% CO2.
DMS114 culture medium Final concentration in media Quantity or Volume
added to 500 mL media
RPMI-1640 NA 500 mL Penicillin-streptomycin solution 100 units/mL penicillin, 50 µg/mL streptomycin 5 mL HEPES 25 mM 7.5 mL FBS 10% v/v 50 mL RPMI-BSA
Add 100 µL of BSA from the bottle containing 30% BSA solution to 30 mL of serum-free RPMI-1640 (at room temperature) in a sterile 50 mL tube. Mix gently to avoid air bubbles. All these procedures should be performed in the laminar flow hood. We make fresh RPMI-BSA before each experiment. Store at room temperature for the duration of the assay.
DMS114 culture medium Final concentration in media Quantity or Volume RPMI-1640 NA 30 mL 30% BSA solution 0.1% v/v 50 µL
Laboratory supplies
NuncTM EasYFlaskTM T-75 cell culture flasks (Thermo Scientific, catalog number: 156499)
Sterile Corning 24-well polystyrene tissue culture insert companion plate with lid (Corning Life Sciences, catalog number: 353504)
96-well plate, non-treated surface, pack of 25 (Thermo Scientific, catalog number: 12-566-202)
PuritanTM sterile DNA-free standard cotton swab, wood handle (Fisherbrand, catalog number: 22-025-201)
Sterile 15 mL polypropylene centrifuge tubes (Thermo Scientific, Nunc, catalog number: 12-556-017)
1.5 mL natural microcentrifuge tubes (Fisherbrand Premium, catalog number: 05-408-129). Autoclave before use
1.5 mL amber-colored microcentrifuge tubes (Fisherbrand Premium, catalog number: 05-408-134). Autoclave before use
Nunc 15 mL conical sterile polypropylene centrifuge tubes with plastic racks (Thermo Scientific, catalog number: 12-565-269)
Nunc 50 mL conical sterile polypropylene centrifuge tubes with plastic racks (Thermo Scientific, catalog number: 12-565-271)
Low-retention 2 mL microcentrifuge tubes (Fisherbrand, catalog number: 02-681-332)
Curved medium point stainless-steel general-purpose forceps (Fisherbrand, catalog number: 16-100-110). Autoclave the forceps before use with the Boyden chamber assay
Fine point high-precision forceps (Fisherbrand, catalog number: 22-327379). Autoclave the forceps before use with the Boyden chamber assay
Equipment
NU-540 laminar-flow biosafety cabinet (NuAire, Plymouth, MN, model: LabGard® ES NU-540 Class II, Type A2)
Cell culture incubator maintained at 37 °C and 5% CO2 (Thermo Scientific, Waltham, MA, model: Heracell VIOS 150i)
Microplate reader (Agilent, model: BioTek Gen 5)
Benchtop centrifuge (ThermoElectron Corporation, model: IEC Centra CL2)
Gilson PIPETMAN Pipettes [Marshall Scientific, catalog number: F144059M (P1000), F144058M (P200), F144056M (P10), F144054M (P2)]
Software and datasets
Agilent BioTek Gen 5 microplate reader and imager software
GraphPad Prism version 10.2.1
Procedure
文章信息
稿件历史记录
提交日期: Apr 10, 2024
接收日期: Jun 30, 2024
在线发布日期: Aug 2, 2024
出版日期: Aug 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Brown, K. C., Sugrue, A. M., Modi, K. J., Light, R. S., Conley, K. B., Cox, A. J., Bender, C. R., Miles, S. L., Valentovic, M. A. and Dasgupta, P. (2024). An Experimental Protocol for the Boyden Chamber Invasion Assay With Absorbance Readout. Bio-protocol 14(15): e5040. DOI: 10.21769/BioProtoc.5040.
分类
癌症生物学 > 侵袭和转移 > 细胞生物学试验 > 细胞侵袭
细胞生物学 > 基于细胞的分析方法 > 酶学测定
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link