- EN - English
- CN - 中文
Slot Blot Analysis of Intracellular Glyceraldehyde-Derived Advanced Glycation End Products Using a Novel Lysis Buffer and Polyvinylidene Difluoride Membrane
新型裂解缓冲液和聚偏二氟乙烯膜在细胞内甘油醛衍生晚期糖基化终产物的点杂交分析中的应用
发布: 2024年07月20日第14卷第14期 DOI: 10.21769/BioProtoc.5038 浏览次数: 1832
评审: Alessandro DidonnaAnonymous reviewer(s)
Abstract
Advanced glycation end products (AGEs) are formed through the reaction/modification of proteins by saccharides (e.g., glucose and fructose) and their intermediate/non-enzymatic products [e.g., methylglyoxal and glyceraldehyde (GA)]. In 2017, Dr. Takanobu Takata et al. developed the novel slot blot method to quantify intracellular GA-derived AGEs (GA-AGEs). Although the original method required nitrocellulose membranes, we hypothesized that the modified proteins contained in the AGEs may be effectively probed on polyvinylidene difluoride (PVDF) membranes. Because commercial lysis buffers are unsuitable for this purpose, Dr. Takata developed the slot blot method using an in-house-prepared lysis buffer containing 2-amino-2-hydromethyl-1,3-propanediol (Tris), urea, thiourea, and 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) that effectively probes AGEs onto PVDF membranes. The slot blot method also entails the calculation of Tris, urea, thiourea, and CHAPS concentrations, as well as protein and mass to be probed onto the PVDF membranes. GA-AGE-modified bovine serum albumin (BSA, GA-AGEs-BSA) is used to draw a standard curve and perform neutralization against a non-specific combination of anti-GA-AGEs antibodies, thereby enabling the quantification of GA-AGEs in cell lysates. This paper presents the detailed protocol for slot blot analysis of intracellular GA-AGE levels in C2C12 cells.
Key features
• This protocol leverages the idea that advanced glycation end products are modified proteins.
• The lysis buffer containing Tris, urea, thiourea, and CHAPS enables probing proteins onto PVDF membranes.
• Intracellular GA-AGE levels may be quantified for some cell types using polyclonal anti-GA-AGE antibodies and standard GA-AGE-modified BSA.
• The lysis buffer may be simultaneously prepared with the cell lysate.
• There is no limit to the type of cultured cells used in the preparation of cell lysate.
Keywords: Advanced glycation end product (晚期糖基化终产物)Graphical overview
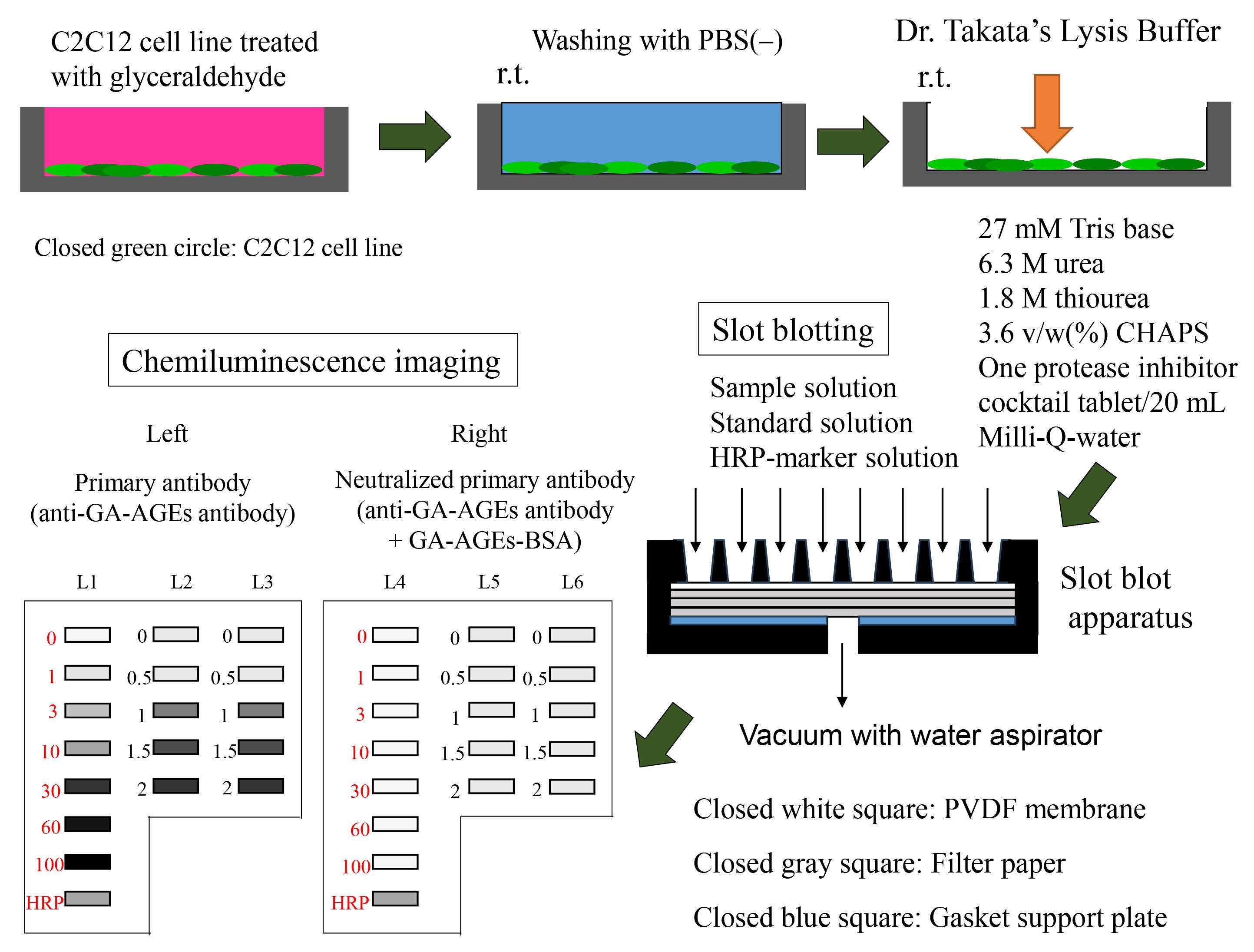
Preparation of sample and slot blot analysis with Dr. Takata’s lysis buffer and polyvinylidene difluoride (PVDF) membranes.
Background
The quantification of intracellular advanced glycation end products (AGEs) is particularly useful in the fields of biochemistry, molecular biology, and protein engineering [1]. AGEs are a type of modified proteins that can be extracted from cultured cells and tissues [2] and indirectly measured using the enzyme-linked immunosorbent assay (ELISA) [3,4]. However, some researchers prefer using a slot blot approach [5–7], because identifying or quantifying some types of AGEs may be difficult using ELISA. Previous slot blot methods for the quantification of AGEs were limited by their use of nitrocellulose membranes and radioimmunoprecipitation (RIPA) buffer [5,6]. Although nitrocellulose membranes are useful in column chromatography, polyvinylidene difluoride (PVDF) membranes are more durable and show greater protein adsorption ability [8,9]. However, there is a lack of suitable lysis buffers for probing proteins onto PVDF membranes. Although RIPA buffer is used in western blotting, it is less suitable in slot blot analyses with PVDF membranes [8,9]. Consequently, Dr. Takanobu Takata developed a lysis buffer containing 2-amino-2-hydromethyl-1,3-propanediol (Tris), urea, thiourea, and 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) to quantify intracellular AGEs using the slot blot analysis [8]. The improved probing efficacy of this lysis buffer may be related to protein carbamoylation [8,9] and the absence of Triton-X, which can inhibit the probing of proteins onto PVDF membranes [8,9]. In 2017, the novel slot blot method was used to accurately quantify intracellular glyceraldehyde (GA)-derived AGEs (GA-AGEs) [10]. From 2017 to 2022, Takata et al. applied this method in the quantification of intracellular GA-AGEs in cells and tissue lysates of the pancreas [10,11], heart [12,13], skeletal muscles [14], liver [15–19], and bone [20] using (i) the novel lysis buffer, (ii) standard GA-AGEs modified bovine serum albumin (BSA), and (iii) neutralization using anti-GA-AGEs antibodies. In this method, the standard GA-AGEs-BSA is used to estimate standard curves [10]; neutralization using a non-specific combination of polyclonal anti-GA-AGEs antibodies [21] avoids biases in the quantification of GA-AGEs [8, 10–17]. We previously applied this protocol to a type of GA-AGEs [10–17] known as toxic AGEs (TAGE) [21]. However, our slot blot may be applied to various types of AGEs, including 1,5-anhydro-fructose AGEs [22] and modified proteins [8,9], even though various AGE modifications may occur in one protein type or molecule [23]. This study presents a validated protocol for the slot blot analysis of intracellular GA-AGE levels using Dr. Takata’s lysis buffer and a PVDF membrane.
Materials and reagents
Biological materials
C2C12 cell line (KAC, Kyoto, catalog number: EC91031101-F0)
The C2C12 cell line is an immortalized mouse myoblast cell line (ATCC, catalog number: CRL1772)
Reagents
Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, catalog number: D6046-500M)
Penicillin/streptomycin solution (FUJIFILM Wako Pure Chemical Corporation, catalog number: 168-23191)
Fetal bovine serum (FUJIFILM Wako Pure Chemical, catalog number: 554-04855)
Glyceraldehyde (Nacalai Tesque, catalog number: 17014-81)
Phosphate-buffered saline (PBS) without Ca2+ and Mg2+ [PBS(-)], 20× (LSI Medience, catalog number: PM102-PN)
2-amino-2-hydromethyl-1,3-propanediol (Tris base) (Tris) (FUJIFILM Wako Pure Chemical, catalog number: 011-20095)
Urea (FUJIFILM Wako Pure Chemical, catalog number: 217-01215)
Thiourea (FUJIFILM Wako Pure Chemical, catalog number: 206-17355)
CHAPS (DOJINDO, Kumamoto, Japan, catalog number: 349-04722)
Methanol (FUJIFILM Wako Pure Chemical, catalog number: 131-01826)
Protease Inhibitor cocktail cOmplete Tablets EDTA-free, EASY pack (Roche, catalog number: 04-693-132-001)
BSA fraction IV (FUJIFILM Wako Pure Chemical, catalog number: 019-2329)
Bradford dye reagent (Takara Bio, catalog number: T9310A-1)
Skim milk for immunoassay (Nacalai Tesque, catalog number: 31149-75)
Tween-20 (GE Healthcare, catalog number: 17-1316-01)
Polyclonal anti-GA-AGE antibodies (purchased from Prof. Masayoshi Takeuchi, Department of Advanced Medicine, Medical Research Institute, Kanazawa Medical University, Uchinada, Japan; 920-0293).
Note: Prof. Takeuchi successfully prepared the polyclonal antibody for TAGE, despite having limited information on the structure of TAGE [21]. Takeuchi et al. reported the hypothetical structure of TAGE in 2023 [24]. The antibody is preserved at -80 °C.
GA-AGE-BSA, 10 mg/mL (or TAGE-BSA, purchased from Prof. Takeuchi)
Note: Despite limited information on the intra- and intermolecular structure of TAGE-BSA, the polyclonal anti-GA-AGE antibody (Reagent 17) developed by Prof. Takeuchi could be probed against the antigen recognition site in TAGE-BSA [21]. The GA-AGEs-BSA was dissolved in PBS and preserved at -30 °C.
Horseradish peroxidase (HRP)-conjugated molecular weight marker (Bionexus, catalog number: BNPM41)
Polyclonal goat anti-rabbit immunological HRP-conjugated antibody (DAKO, catalog number: REF0448)
ImmunoStar LD kit (FUJIFILM Wako Pure Chemical, catalog number: 292-69903)
Milli-Q ultrapure water
Note: Purchased with an RFU554CA ultrapure water system (Advantech Toyo, Tokyo, Japan) at the Kanazawa Medical University.
Solutions
Medium for cell culture (see Recipes)
PBS(-) 1× (see Recipes)
Solution A (see Recipes)
Solution B (see Recipes)
Solution C (see Recipes)
Solution D (see Recipes)
20 mg/mL BSA in Solution D (20 mg/mL BSA solution) (see Recipes).
Diluted BSA solution (see Recipes).
Diluted GA-AGEs-BSA solution (see Recipes)
HRP-conjugated molecular weight marker solution (see Recipes)
PBS-T (see Recipes)
5% SM-PBS-T (see Recipes)
0.5% SM-PBS-T (see Recipes)
Primary antibody solution (see Recipes)
Neutralized primary antibody solution (see Recipes)
Secondary antibody solution (see Recipes)
Recipes
Medium for cell incubation
Mix the reagents on a laminar flow hood.
Note: Because this step can easily be performed in-house prior to the experiment, we do not provide a detailed description here. Complete information may be found in our previous studies [10–16]. Media can be stored in the general refrigerator (4 °C).
Reagent Volume Recommended storage condition DMEM 450 mL 4 °C Penicillin/streptomycin solution 5 mL -30 °C Fetal bovine serum 50 mL -30 °C Total 505 mL PBS(-) (1×)
Room temperature refers to 22–28 °C.
Reagent Volume Recommended storage condition PBS(-) (20×) 50 mL Room temperature Milli-Q water 950 mL Room temperature Total 1,000 mL Solution A
Using a 50 mL polypropylene centrifuge tube and serological pipet, dissolve Tris in Milli-Q water (10 mL) to prepare 1 mol/L (M) Tris solution. Solution A may be stored at -30 °C for 6 months.
Note: The pH of the Tris solution was approximately 9.4. Only Tris powder was dissolved in Milli-Q-water, without adding other reagents (e.g., hydrochloride).
Reagent Quantity/volume Recommended storage condition Final concentration Tris 1.21 g Room temperature 1 mol/L (M) Milli-Q water 10 mL Room temperature Total 10 mL Solution B
Dissolve one tablet of protease inhibitor cocktail (cOmplete Tablets EDTA free) in Milli-Q water (2 mL) within a microcentrifuge tube; transfer the Milli-Q water to the tube using a 100–1,000 μL Gilson PIPETMAN. We recommend Solution B (2 mL) to be prepared and used immediately with every experiment. However, Solution B can be preserved at -30 °C for three months if it is reused. Approximately 100 μL of Solution B were preserved.
Reagent Quantity/Volume Recommended storage condition Final concentration Protease inhibitor cocktail 1 tablet 4 °C 1 tablet/2 mL Milli-Q water 2 mL Room temperature Total 2 mL Solution C
Solution C contains 30 mM Tris, 7 M urea, 2 M thiourea, and 4% CHAPS. Mix these reagents in a 50 mL polypropylene centrifuge tube and add Milli-Q water to a final volume of 20 mL. Treat 1 M Tris with Milli-Q water using a 200–1,000 μL Gilson PIPETMAN.
Note: The reagents are added to the centrifuge tube in no specific order. At room temperature, urea and thiourea are not easily dissolved in Milli-Q water; accordingly, we recommend using a vortex system (e.g., Vortex-Genie 2) at room temperature. We do not recommend dissolution in a 37 °C CO2 incubator or water bath because urea and thiourea may produce cyanate or isocyanic acid at high temperatures [9]. Solution C can be preserved at -30 °C for three months, though we recommend preparing it immediately before use.
Reagent Quantity/Volume Recommended storage condition Final concentration 1 M Tris 0.6 mL Room temperature 30 mM Urea 8.40 g Room temperature 7 M Thiourea 3.04 g Room temperature 2 M CHAPS 0.80 g Room temperature 4 w/v (%) Milli-Q water 20 mL Room temperature Total 20 mL Solution D
Prepare Solution D by mixing Solutions B and C at a ratio of 1:9. Transfer Solution C using the 200–1,000 μL Gilson PIPETMAN. Transfer Solution D using a 10 mL serological pipette. Solution D contains 27 mM Tris, 6.3 M urea, 1.8 M thiourea, and 3.6 v/w (%) CHAPS.
Note: We recommend dissolution using a vortex system (e.g., Vortex-Genie 2) at room temperature. Solution D can be preserved at -30 °C for three months, though we recommended preparing it immediately before use. Solution D was used as lysis buffer to prepare the cell lysate [8–17], which was prepared from C2C12 cells treated with glyceraldehyde [14]. Although “Modified Solution C” containing 30 mM Tris, 7 M urea, 2 M thiourea, 4% CHAPS, and 4% Solution B may also be used as lysis buffer [18–20,22], we recommend that Solution D be used because it contains sufficient urea, thiourea, and CHAPS to generate the carbamylated AGE proteins [8,9]. The cell lysates and unused Solution D should be preserved at -30 °C until later use in the slot blot experiment. The condition of the unused Solution D and the cell lysates should be arranged.
Reagent Volume Final concentration Solution B 2 mL 10% Solution C 18 mL 90% Total (optional) 20 mL BSA–Solution D mixture (20 mg/mL BSA solution)
Dissolve 20 mg of BSA in Solution D (10 mL) within a 50 mL polypropylene centrifuge tube. Transfer Solution D using a 200–1,000 μL Gilson PIPETMAN.
Note: BSA cannot be easily dissolved in Solution D; thus, we recommend using a vortex system (e.g., Vortex-Genie 2) at room temperature. The presence of undissolved BSA can be confirmed by the presence of a gelatinous substance. Dissolution by vortex should not be performed at high temperatures (e.g., 37 °C) [9].
Note: We do not recommend commercial BSA for use in this experiment. An in-house solution should preferably be prepared. We believe that BSA fraction IV is suitable for the Bradford method in our protocol.
Reagent Quantity/Volume Final concentration Recommended storage condition BSA 20 mg 2.0 mg/mL (μg/μL) 4 °C Milli-Q water 10 mL Room temperature Total 10 mL Diluted BSA solution
Dilute 20 mg/mL BSA solution to 0.0625, 0.125, 0.25, 0.5, 1.0, and 1.5 μg/μL in a 1.5 mL microcentrifuge tube; transfer BSA using a 50–200 μL Gilson PIPETMAN. We recommend that dissolution be performed using a vortex system (e.g., Vortex-Genie 2) at room temperature. The BSA solution should be made and used immediately with every experiment. BSA solution should be aliquoted (approximately 100 μL) and preserved at -80 °C if it will be reused. However, we recommend preparing and using a fresh BSA solution per experiment.
Note: Prepare from 20 mg/mL BSA solution (Recipe 7).
Volume of reagents Volume of Solution D Total volume Final concentration 150 μL of 2.0 μg/μL BSA solution 50 μL 200 μL 1.5 μg/μL 100 μL of 2.0 μg/μL BSA solution 100 μL 200 μL 1.0 μg/μL 100 μL of 1.0 μg/μL BSA solution 100 μL 200 μL 0.5 μg/μL 100 μL of 0.5 μg/μL BSA solution 100 μL 200 μL 0.25 μg/μL 100 μL of 0.25 μg/μL BSA solution 100 μL 200 μL 0.125 μg/μL 100 μL of 0.125 μg/μL BSA solution 100 μL 200 μL 0.0625 μg/μL Diluted GA-AGEs-BSA solution
Dilute 10 mg/mL GA-AGEs-BSA in PBS(-) within a 1.5 mL microcentrifuge tube; transfer the solution using a 50–200 μL or 1–10 μL Gilson PIPETMAN.
Note: We recommend the diluted GA-AGEs-BSA solution to be prepared before being used.
Volume of reagents Volume of PBS(-) Total volume Final concentration 2.0 μL of 10 mg/mL GA-AGEs-BSA 98 μL 100 μL 200 ng/μL 20 μL of 200 ng/μL GA-AGEs-BSA 60 μL 80 μL 50 ng/μL 60 μL of 50 ng/μL GA-AGEs-BSA 40 μL 100 μL 30 ng/μL 30 μL of 50 ng/μL GA-AGEs-BSA 70 μL 100 μL 15 ng/μL 10 μL of 50 ng/μL GA-AGEs-BSA 90 μL 100 μL 5 ng/μL 10 μL of 15 ng/μL GA-AGEs-BSA 90 μL 100 μL 1.5 ng/μL 10 μL of 5 ng/μL GA-AGEs-BSA 90 μL 100 μL 0.5 ng/μL HRP-conjugated molecular weight marker solution
Dilute the HRP-conjugated molecular weight marker in PBS(-) within a 1.5 mL microcentrifuge tube; transfer the solution using the 200–1,000 μL or 1–10 μL Gilson PIPETMAN.
Note: We recommend dissolution to be performed using a vortex system (e.g., Vortex-Genie 2) at room temperature and the diluted GA-AGEs-BSA solution to be prepared at the time of use.
Reagent Volume Recommended storage condition HRP-conjugated molecular weight marker 3 μL -30 °C PBS(-) 197 μL Room temperature Total 200 μL PBS-T
Dissolve Tween-20 in PBS(-). Transfer Tween-20 using the 200–1,000 μL Gilson PIPETMAN.
Reagent Volume Recommended storage condition Tween-20 0.5 mL Room temperature PBS(-) 1,000 mL Room temperature Total 1,000.5 mL 5% SM-PBS-T for immunoassay
Dissolve skim milk for immunoassay in PBS-T within a 50 mL polypropylene centrifuge tube; transfer PBS-T using the 10 mL serological pipette.
Note: We recommend that dissolution be performed using a vortex system (e.g., Vortex-Genie 2) at room temperature and that 5% SM-PBS-T be prepared before use. However, the solution may be preserved at 4 °C for two days.
Reagent Quantity/Volume Recommended storage condition Skim milk for immunoassay 2.5 g 4 °C PBS-T 50 mL Room temperature Total 50 mL 0.5% SM-PBS-T
Dilute 5% SM-PBS-T to 0.5% SM-PBS-T in a 50 mL polypropylene centrifuge tube; transfer PBS-T using the 10 mL serological pipette.
Note: We recommend that dissolution be performed using a vortex system (e.g., Vortex-Genie 2) at room temperature and that 5% SM-PBS-T be prepared before use, which may occur at room temperature. However, the solution may be preserved at 4 °C for two days.
Reagent Volume Recommended storage condition 5% SM-PBS-T 5 mL Room temperature PBS-T 45 mL Room temperature Total 50 mL Primary antibody solution
Mix 5 mL of 0.5% SM-PBS-T (Recipe 13) using a 10 mL serological pipette with 5 μL of polyclonal anti-GA-AGE antibody (using a 2–20 μL Gilson PIPETMAN) within a 15 mL polypropylene centrifuge tube.
Note: We recommend that dissolution be performed using a vortex system (e.g., Vortex-Genie 2) for 10 s at room temperature. The polyclonal anti-GA-AGE antibody, which was preserved at -80 °C, should first be defrosted at 0 °C (in ice) and may then be preserved at 4 °C for half a year.
Reagent Volume Recommended storage condition Dilution ratio Polyclonal anti-GA-AGEs antibody 5 μL -80 °C 1:1,000 0.5% SM-PBS-T 5.0 mL (5,000 μL) Room temperature Total (optional) 5.005 mL (5,005 μL) Neutralized primary antibody solution
Mix 5 μL of polyclonal anti-GA-AGEs antibody (2–20 μL Gilson PIPETMAN) with 125 μL of 10 mg/mL GA-AGEs-BSA (50–200 μL Gilson PIPETMAN) and 4.875 mL of 0.5% SM-PBS-T (10 mL serological pipette) within a 15 mL polypropylene centrifuge tube.
Note: We recommend that dissolution be performed using a vortex system (e.g., Vortex-Genie 2) for 10 s at room temperature. Polyclonal anti-GA-AGEs and GA-AGEs-BSA were preserved at -80 °C and -30 °C, respectively, and defrosted on ice. We recommend that 10 mg/mL GA-AGEs-BSA be divided into 130 μL aliquots and preserved at -30 °C in advance. Moreover, we recommended the divided and preserved GA-AGEs-BSA to be used, and one of them should be for one experiment (they should not be refrozen and reused).
Reagent Volume Recommended storage condition Dilution ratio/Final concentration Polyclonal anti-GA-AGEs antibody 5 μL -80 °C 1:1,000 10 mg/mL GA-AGEs-BSA 125 μL -30 °C 1/40 (250 μg/mL) 0.5% SM-PBS-T 4.875 mL (4,875 μL) Room temperature Total 5.005 mL (5,005 μL) Secondary antibody solution
Mix 5 mL of 0.5% SM-PBS-T (10 mL serological pipette) with 2.5 μL of polyclonal goat anti-rabbit immunological HRP-conjugated antibody (1–10 μL Gilson PIPETMAN) within a 15 mL polypropylene centrifuge tube. We recommend that dissolution be performed using a vortex system (e.g., Vortex-Genie 2) for 10 s at room temperature.
Reagent Volume Recommended storage condition Dilution ratio Polyclonal goat anti-rabbit immunological HRP-conjugated antibody 2.5 μL 4 °C 1:2,000 0.5% SM-PBS-T 5 mL (5000 μL) Room temperature Total 5.0025 mL (5002.5 μL)
Laboratory supplies
60 mm dish (BM Equipment, catalog number: 93060)
1.5 mL microcentrifuge tube (Thermo Fisher Scientific, catalog number: 3451)
2.0 mL microcentrifuge tube (Watson, catalog number: 132-6201)
50 mL polypropylene centrifuge tube (TrueLine; Nippon Genetics, catalog number: TR2004)
15 mL polypropylene centrifuge tube (TrueLine; Nippon Genetics, catalog number: TR2000)
10 mL serological pipette (BM Equipment, catalog number: 207500-SLP-10)
Rubber bulb for 10 mL serological pipette (AS ONE, catalog number: 6-356-4)
Disposable polypropylene tray (AS ONE, catalog number: 1-3145-03)
Dispenser (TPP, BM Equipment, catalog number: 99010)
1–10 μL pre-sterilized tip (10 μL) (BM Equipment, catalog number: W10-RS)
50–200 μL pre-sterilized tip (200 μL) (Watson, catalog number: 62-0887-34)
200–1,000 μL pre-sterilized tip (1,000 μL) (Watson, catalog number: 38688239)
96-well microplates (Becton Dickinson, catalog number: REF353072)
25 mL reagent reservoir (BM Equipment, catalog number: BM-0850-1)
PVDF membrane (pore size: 0.45 μm) (Merck Millipore, catalog number: IPVH00010)
Filter paper (9 cm × 12 cm) (Bio-Rad Laboratories, catalog number: 1620161)
Hybri-Bag (hard type) (Cosmo Bio, catalog number: S1001)
Gilson PIPETMAN (GILSON, model type: 100–1,000 μL)
Gilson PIPETMAN (GILSON, model type: 50–200 μL)
Gilson PIPETMAN (GILSON, model type: 2–20 μL)
Gilson PIPETMAN (GILSON, model type: 1–10 μL)
Nichipet 7000 range: 50–200 μL (Nichiryo, Tokyo, Japan)
Equipment
Ultrapure Water System (Advance Toyo, model: RFU554CA)
Flask-trap aspirator (1,000 mL) (Biosan, Riza, Latvia; model number: FTA-1)
Centrifuge (Eppendorf, model: 5415R)
Microplate reader (Bio-Rad, model: iMark)
Vortex-Genie 2 (M&S Instruments, catalog number: 33230217)
Imager (M&S Instruments, model: Fusion FX)
AS-200 Heat sealer (AS ONE, catalog number: H221049/01167C)
Seesaw shaker (BIO CRAFT, Tokyo, model: BC-700)
Aspirator with water pump (AS ONE, catalog number: 1-689-02)
Bio-Dot SF microfiltration apparatus (48 lanes) (Bio-Rad; Figure 1)
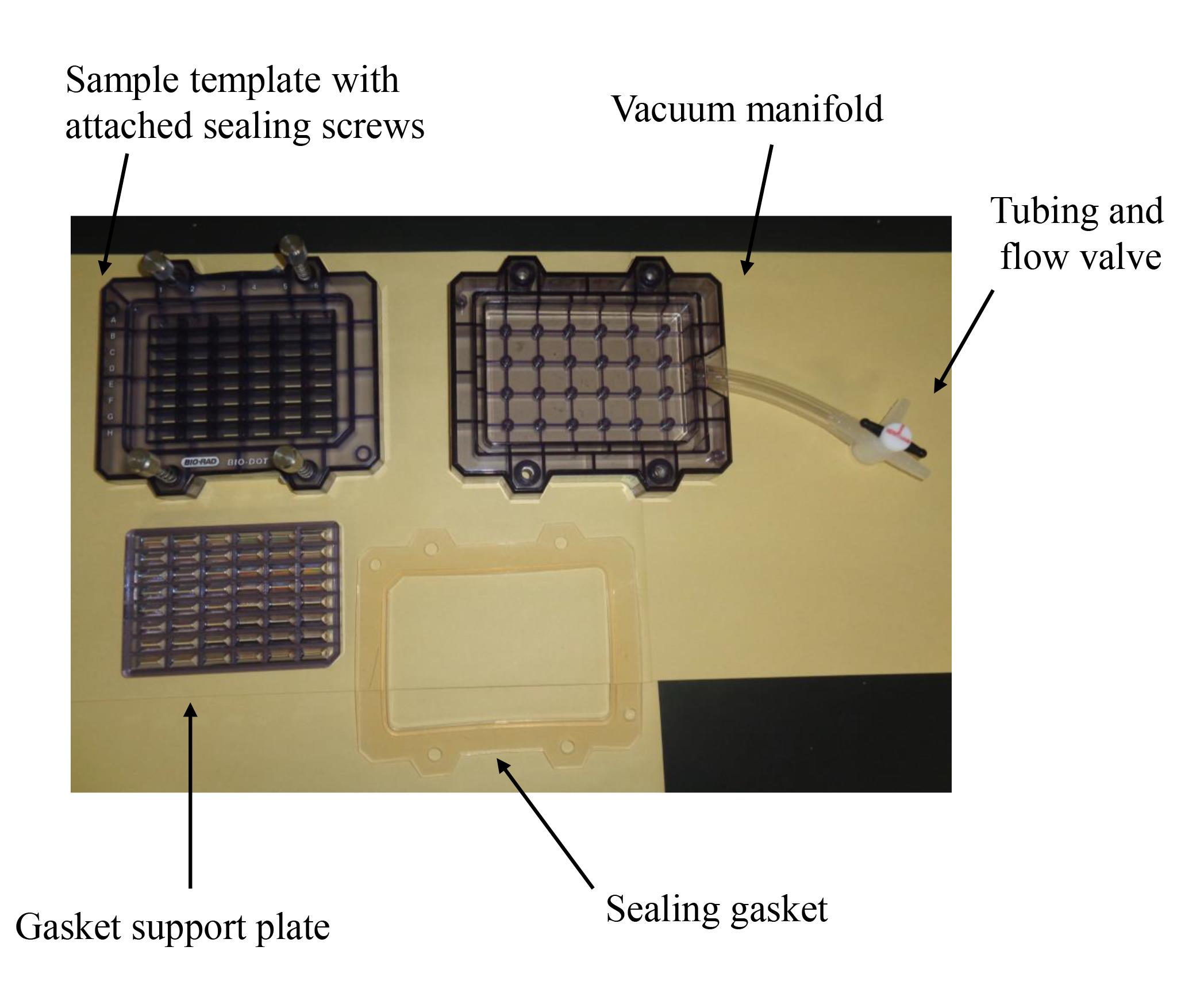
Figure 1. Bio-Dot SF microfiltration apparatus (48 lanes) containing sample template and equipped with sealing screws, sealing gasket, gasket support plate, vacuum manifold, and tubing with flow valve
Software and datasets
Excel software (Microsoft, Redmond, WA, USA. version 2010, 2013, 2016)
Note: The software was installed on a standard personal computer (OS: Windows 7, 10).
FUSION FX software (M&S Instruments, version: 17.03)
Note: The software was installed on a standard personal computer (OS: Windows 7) with Fusion FX imager (M&S Instruments) and was used since November 2017 at Kanazawa Medical University [11,13–16,22]. However, other chemiluminescence imagers and software may be used [10,12,17–20].
Stat FX software (Artech, Osaka, Japan, version: 6)
Note: When the slot blot analysis was performed in more than three independent experiments, Statflex (version 6) was used to perform statistical analysis [10–16,18–20].
Procedure
文章信息
稿件历史记录
提交日期: Apr 6, 2024
接收日期: Jun 23, 2024
在线发布日期: Jul 5, 2024
出版日期: Jul 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Takata, T., Murayama, H. and Masauji, T. (2024). Slot Blot Analysis of Intracellular Glyceraldehyde-Derived Advanced Glycation End Products Using a Novel Lysis Buffer and Polyvinylidene Difluoride Membrane. Bio-protocol 14(14): e5038. DOI: 10.21769/BioProtoc.5038.
分类
生物化学 > 糖类 > 葡萄糖
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link