- EN - English
- CN - 中文
Purification and Cryo-Electron Microscopy Analysis of Bacterial Appendages
细菌附属物的纯化与冷冻电镜分析
发布: 2024年07月20日第14卷第14期 DOI: 10.21769/BioProtoc.5032 浏览次数: 2812
评审: Munenori IshibashiElena A. OstrakhovitchAnonymous reviewer(s)
Abstract
A number of extracellular helical protein polymers are crucial for supporting bacterial motility. The bacterial flagellum is a polymeric appendage used to support cellular motility. Historically, structural studies of flagellar and other filaments were limited to those present as or locked into straightened states. Here, we present a robust workflow that produces biologically relevant high-resolution cryo-electron microscopy (cryo-EM) structures of bacterial flagellar filaments. We highlight how a simple purification method, centered around several centrifugation steps, exploits the process of filament ejection in Caulobacter crescentus and results in isolated filaments amenable to transmission electron microscopy (TEM) studies. The quality of the sample is validated by SDS-PAGE and negative stain TEM analysis before a sample is vitrified for cryogenic electron microscopy (cryo-EM) data collection. We provide a detailed protocol for reconstructing either straight or curved flagellar filaments by cryo-EM helical reconstruction methods, followed by an overview of model building and validation. In our hands, this workflow resulted in several flagellar structures below 3 Å resolution, with one data set reaching a global resolution of 2.1 Å. The application of this workflow supports structure-function studies to better understand the molecular interactions that regulate filament architecture in biologically relevant states. Future work will not only examine interactions that regulate bacterial flagellar and other filament organization but also provide a foundation for developing new helical biopolymers for biotech applications.
Key features
• Rapid high-quality purification of bacterial flagella via simple bacterial culturing, centrifugation, and resuspension methods.
• High-throughput cryo-EM data collection of filamentous objects.
• Use of cryoSPARC implementations of helical reconstruction algorithms to generate high-resolution 3D structures of bacterial flagella or other helical polymers.
Keywords: Cryo-EM (冷冻电镜)Graphical overview
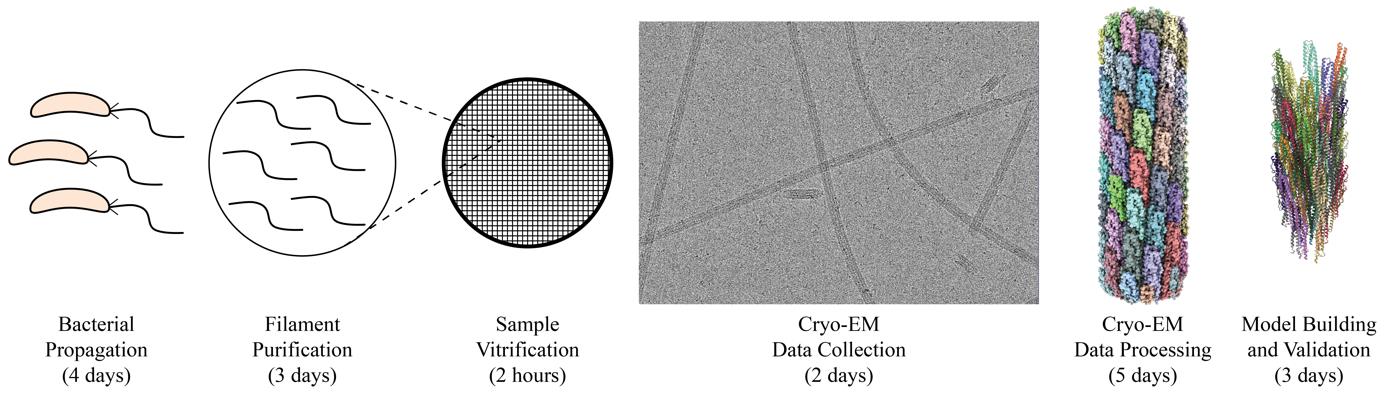
Graphical overview of flagellar filament purification and cryo-EM imaging and analysis. Following bacterial propagation of C. crescentus, cells are pelleted, and a series of centrifugation steps are used to collect ejected flagellar filaments. The filament quality is assessed before the sample is vitrified for cryo-EM data collection. Cryo-EM data is processed generating an electron potential map that is used for building an atomic model of the filament.
Background
The flagellum is an appendage that supports motility in many bacterial species. The flagellum, an extracellular helical propeller, provides cells with a means to navigate through viscous environments. At the cell membrane, an ion-powered motor complex generates torque to rotate the helical filament. Although critical for cellular function, flagellum synthesis is an energetically expensive process that requires the regulated expression of over 60 genes to form a fully functional flagellum. These genes encode both structural and regulatory proteins [1,2]. Bacterial flagella are complex nanomachines, which have evolved to support the lifestyles and ecological niches of the associated species. It has been determined that ~45% of bacterial species possess multiple flagellin proteins, the structural proteins that comprise the flagellar filament [3,4]. In some species, only a single flagellin is synthesized and incorporated into the nascent filament due to phase variation, while others generate filaments comprised of multiple different flagellins [5–8]. Previously, studying the structure of these flexible helical polymers would require the introduction of point mutations to lock the flagellar filament into straightened forms [9,10]. Through these studies, it was determined that the flagellum was organized into 11 protofilaments made up of many flagellin monomers, each of which contains four domains, denoted D0, D1, D2, and D3 (Figure 1A). However, these studies were limited because little information could be gleaned about biologically relevant molecular interactions that impact flagellin subunit packing and filament architecture, i.e., without the imposed straightening mutations.
Our studies focus on the dimorphic bacterium, Caulobacter crescentus. The C. crescentus genome contains six flagellin genes that are expressed and incorporated into the wild-type filament [3,5]. Our structural analyses have shown that C. crescentus flagellins lack the D2 and D3 domains (Figure 1B) [11]. Interestingly, as C. crescentus undergoes cell division, a cell will eject the polar flagellar filament to allow for the synthesis of a polar stalk. The filament purification method presented here concentrates ejected filaments through a series of centrifugation and washing steps. The process of filament ejection can be artificially stimulated in other species when cells exhaust nutrients and enter the stationary phase [12,13]. Incorporating this step during bacterial propagation could allow our workflow to be applied to many other bacteria. The purified flagellar filaments were assessed by SDS-PAGE analysis and negative stain transmission electron microscopy (TEM) before cryo-EM studies. Although single-particle cryo-EM structure determination has exploded in the past decade, tools specific to iterative real-space helical reconstruction have lagged [14]. Fortunately, several new tools have become available that accelerate the reconstruction of helical polymers to high resolution [15–20]. Here, we demonstrate the application of those tools to determine the structures of both straight and curved helical polymers below 3.0 Å resolution. The protocol encompasses (1) methods to purify flagellar filaments from exhausted media, (2) detailed cryo-EM reconstruction workflows that use either symmetrized or asymmetrical reconstruction methods to resolve straight or highly curved filaments, and (3) an outline for model building and validation of large multimeric protein models based on the reconstructed maps. The protocols can be applied to studies of flagellar filament structures across multiple species. Additionally, the general cryo-EM workflow is not limited to flagellar filaments and can be applied to other helical biopolymers.
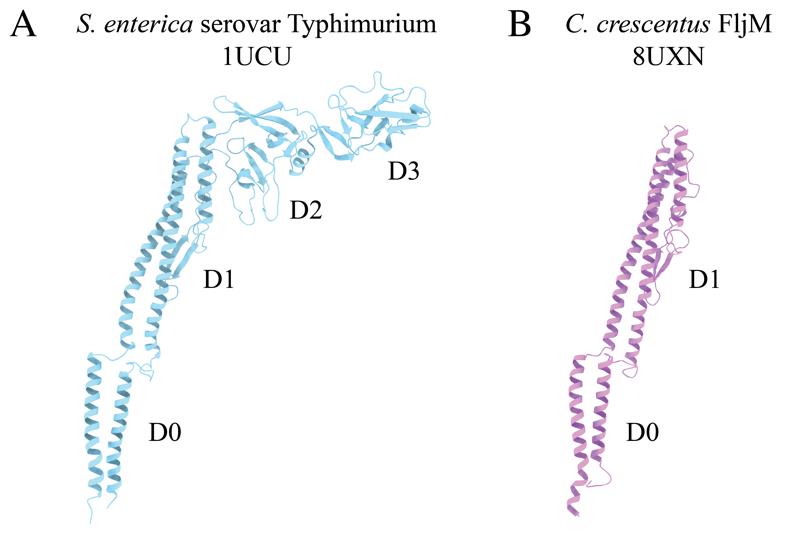
Figure 1. Comparison of bacterial flagellin models. A. Canonical flagellin model from Salmonella enterica serovar Typhimurium with D0, D1, D2, and D3 domains (PDB: 1UCU) [9]. B. Caulobacter crescentus FljM flagellin model with D0 and D1 domains, and lacking D2 and D3 domains (PDB: 8UXN) [21].
Materials and reagents
Biological materials
Caulobacter crescentus ΔfljJKLNO (FljM only); C. crescentus strain with all flagellins deleted and the fljM flagellin gene introduced on a plasmid with kanamycin resistance (ERW2301) [21]
Caulobacter crescentus ΔfljJLMNO (FljK only); C. crescentus strain with genome deletions to all flagellins except for the fljK gene (TPA2353) [3]
Reagents
Peptone (Fisher Scientific, catalog number: BP1420-500)
Yeast extract (Fisher Scientific, catalog number: BP1422-500)
Bacto agar (BD Difco, catalog number: DF0140010)
Magnesium sulfate heptahydrate (Millipore Sigma, catalog number: MX0070)
Calcium chloride dihydrate (Sigma-Aldrich, catalog number: C3306-500G)
Kanamycin sulfate (Gibco, catalog number: 11-815-024)
Protease inhibitor cocktail (Millipore Sigma, catalog number: 539137)
Phosphate-buffered saline (1× PBS) (Corning, catalog number: 21-040-CV)
AnyKD Mini-PROTEIN TGX stain-free protein gels (Bio-Rad, catalog number: 4568126)
PageRuler Plus prestained protein ladder (Thermo Fisher, catalog number: 26619)
Coomassie Brilliant Blue (Sigma-Aldrich, catalog number: B7920-50G)
2% uranyl acetate (UA) (EMS, catalog number: 22400-2)
Solutions
1 M MgSO4 (see Recipes)
1 M CaCl2 (see Recipes)
Peptone yeast extract (PYE) agar (see Recipes)
Peptone yeast extract (PYE) liquid media (see Recipes)
1% Uranyl Acetate (see Recipes)
Recipes
1 M MgSO4
Reagent Final concentration Amount Magnesium sulfate heptahydrate 1 M 61.62 g H2O n/a 250 mL Total n/a 250 mL Dissolve the MgSO4 in water while stirring.
Filter the solution through a 0.22 µm filter sterile bottle top filter.
1 M CaCl2
Reagent Final concentration Amount Calcium chloride dihydrate 1 M 36.75 g H2O n/a 250 mL Total n/a 250 mL Dissolve the CaCl2 in water while stirring.
Filter the solution through a 0.22 µm filter sterile bottle top filter.
Peptone yeast extract (PYE) agar
Reagent Final Concentration Amount Peptone 0.2% (w/v) 2 g Yeast extract 0.1% (w/v) 1 g Bacto agar 1.5% (w/v) 15 g 1 M MgSO4 1 mM 1 mL 1 M CaCl2 0.5 mM 0.5 mL H2O n/a Fill up to 1 L Total n/a 1 L Combine peptone, yeast, Bacto agar, and H2O in a flask and autoclave at 121 °C, 15 PSI for at least 20 min.
Allow the media to cool to ~55 °C before adding the salts.
Note: For antibiotic-resistant strains, the media is supplemented with kanamycin at a concentration of 50 µg/mL.
From 1 L of media, pour approximately 25 mL per plate into approximately 40 Petri plates (100 mm).
Peptone yeast extract (PYE) liquid media
Reagent Final concentration Amount Peptone 0.2% (wt/v) 2 g Yeast extract 0.1% (wt/v) 1 g 1 M MgSO4 1 mM 1 mL 1 M CaCl2 0.5 mM 0.5 mL H2O n/a Fill up to 1 L Total n/a 1 L Combine peptone, yeast, and H2O in a flask and autoclave at 121 °C, 15 PSI for at least 20 min.
Allow the media to cool to ~55 °C before adding the salts.
Note: for antibiotic-resistant strains, the media was supplemented with kanamycin at a concentration of 50 µg/mL.
1% uranyl acetate (UA)
Reagent Final concentration Amount 2% uranyl acetate 1% (v/v) 250 µL H2O n/a 250 µL Total n/a 500 µL Mix 1 part sterile water with 1 part 2% UA stain (EMS) and filter through a Spin-X centrifuge tube with a 0.22 µm filter (Costar).
Laboratory supplies
100 mm × 15 mm Petri dishes (Fisher Scientific, catalog number: S33580A)
250 mL vacuum filtration system (VWR, catalog number: 10040-464)
1.7 mL centrifuge tubes (Denville, catalog number: C2170)
0.22 µm Spin-X centrifuge tube filter (Costar, catalog number: 8160)
200 mesh carbon film, copper grids (EMS, catalog number: CF200-CU)
Parafilm (Bemis, catalog number: PM996)
Whatman #1 filter paper (Whatman, catalog number: 1001-090)
Quantifoil R2/1 200 mesh, copper grids (Quantifoil Micro Tools GmbH, catalog number: Q210CR1)
Standard Vitrobot Filter Paper, Ø55/20 mm, Grade 595 (Ted Pella, catalog number: 47000-100)
Equipment
NanoDrop spectrophotometer (Thermo Fisher, catalog number: ND2000)
Incubator with shaker (Labnet, catalog number: I-5311-DS)
500 mL centrifuge bottles (Nalgene, catalog number: 3141-0500)
70 mL polycarbonate bottle assembly with aluminum caps (Beckman Coulter, catalog number: 355622)
RC-5B Plus centrifuge (Sorvall, catalog number: SO-RC5B)
GS-3 rotor (Sorvall, catalog number: SO-GSA3)
Optima XL-80K ultracentrifuge (Beckman Coulter, catalog number: 8043-30-1211)
Type 45 Ti fixed angle titanium rotor (Beckman Coulter, catalog number: 339160)
Harvard Trip 1400/1500 Series balance (Ohaus, catalog number: 80000005)
5424 R microcentrifuge (Eppendorf, catalog number: 05-400-005)
KS 260 basic shaker (IKA, catalog number: Z341835)
Plasma cleaner (Harrick Plasma Inc., catalog number: PDC-32G)
Static dissipator (Mettler Toledo, catalog number: UX-11337-99)
PTFE well plate (custom made; similar product: Kibron, catalog number: 6344)
Style N5 reverse pressure tweezers (Dumont, catalog number: 0202-N5-PS-1)
Talos L120C 120 kV transmission electron microscope (TEM) (Thermo Fisher Scientific), or equivalent
Cryo grid box (Sub-Angstrom, catalog number: SB)
Vitrobot Mark IV vitrification robot (Thermo Fisher Scientific)
Titan Krios G3i 300 kV transmission electron microscope (TEM) (Thermo Fisher Scientific)
K3-GIF direct electron detector with energy filter (Gatan Inc., AMETEK)
Supermicro server (KingStar) with two Xeon E5-2640 processors (Intel), 256 GB memory, and 4× 1080Ti GPU (NVIDIA), or equivalent setup.
Software and datasets
CryoSPARC (https://cryosparc.com/)
AlphaFold2 (https://alphafold.ebi.ac.uk)
UCSF ChimeraX (https://www.cgl.ucsf.edu/chimerax/)
PHENIX (https://phenix-online.org/)
Coot (https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/)
Procedure
文章信息
稿件历史记录
提交日期: May 6, 2024
接收日期: Jun 14, 2024
在线发布日期: Jun 30, 2024
出版日期: Jul 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Sanchez, J. C., Baumgardt, J. K. and Wright, E. R. (2024). Purification and Cryo-Electron Microscopy Analysis of Bacterial Appendages. Bio-protocol 14(14): e5032. DOI: 10.21769/BioProtoc.5032.
分类
生物物理学 > 显微技术 > 低温显微镜技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link