- EN - English
- CN - 中文
A Protocol for Preparing Mucoadhesive Emulsion Microgels and Assessing Their Mucoadhesion Properties In Vitro
黏附性乳液微凝胶的制备及其体外黏附性评价实验方案
发布: 2024年07月05日第14卷第13期 DOI: 10.21769/BioProtoc.5027 浏览次数: 1228
评审: Olga KopachAmira S HanafyMathilde UllrichAnonymous reviewer(s)
Abstract
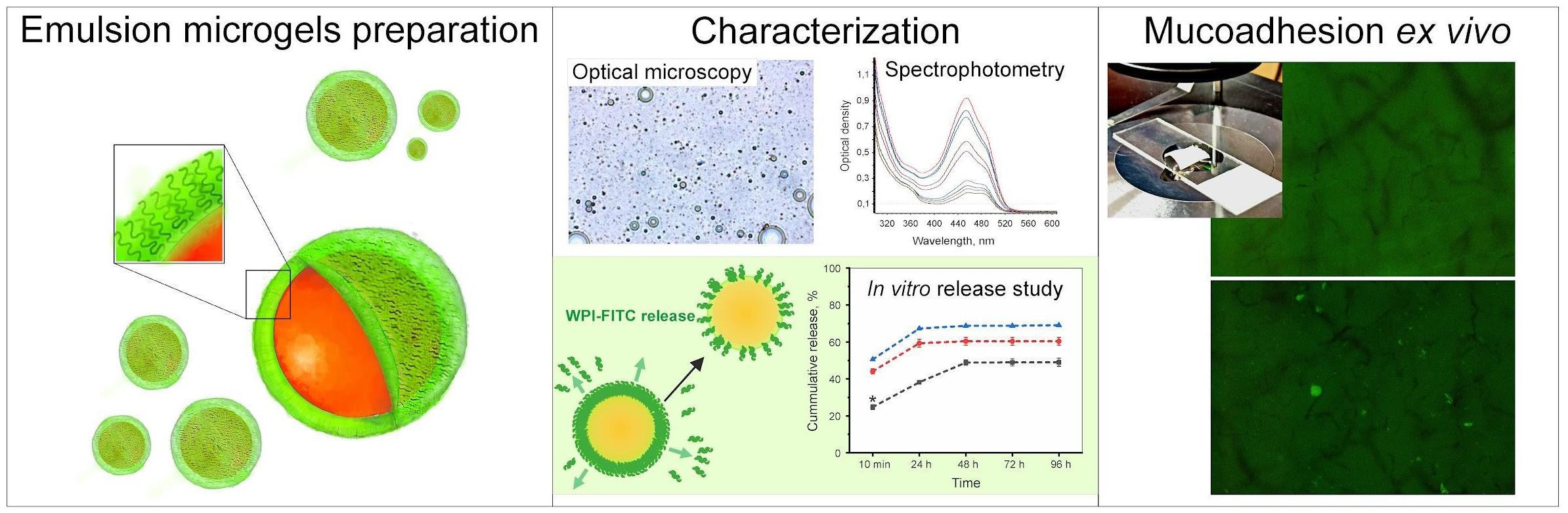
Intravesical instillation is an efficient therapeutic technique based on targeted administration of a drug directly into the lesion for the treatment of bladder diseases. This is an alternative to traditional systemic administration of drugs. However, this technique requires repeated procedures, which can lead to even greater inflammation and infection of the urethra. To date, novel systems that allow prolonged drug retention in the bladder cavity are actively being developed. We recently reported a targeted drug delivery system based on the mucoadhesive emulsion microgels consisting of the natural component whey protein isolate. Such micron-sized carriers possess high loading capacity, a prolonged drug release profile, and efficient mucoadhesive properties to the bladder urothelium. As a continuation of this work, we present a protocol for the synthesis of mucoadhesive emulsion microgels. Detailed procedures for preparing precursor solutions as well as studying the physico-chemical parameters of microgels (including loading capacity and drug release rate) and the mucoadhesive properties using the model of porcine bladder urothelium are discussed. Precautionary measures and nuances that are worth paying attention to during each experimental stage are given as well.
Key features
• The protocol for the synthesis of mucoadhesive emulsion microgels based on whey protein isolate is presented. The experimental conditions of emulsion microgels synthesis are discussed.
• Methods for studying the physico-chemical properties of mucoadhesive emulsion microgels (size of emulsion microgels particles, loading capacity, release kinetics) are described.
• The method for assessing mucoadhesive properties of emulsion microgels is demonstrated using the porcine bladder tissue model ex vivo.
Keywords: Emulsion microgels (EM) (乳液微凝胶)Graphical overview
Background
Currently, for the treatment of urinary system diseases, such as cystitis or bladder cancer, targeted drug delivery using a catheter directly to the site of the disease is widely used. The intravesical instillation of antibacterial or cytostatic drugs has significant advantages over systemic administration; particularly, allowing the reduction of side effects on healthy organs (namely, inducing liver function disorders, undesirable effects on the central nervous system, and blood pressure, as well as delayed mutagenic, teratogenic, and carcinogenic effects of drugs) [1,2]. However, this delivery method has a number of disadvantages as well, which are conditioned primarily by the structure and basic physiological functions of the urinary system. The inner surface of the urinary bladder wall—the so-called urothelium—is represented by umbrella cells, whose size varies depending on their stretching degree. The surface of umbrella cells is covered with glycoproteins and proteoglycans, which form the glycosaminoglycan layer. This layer acts as an efficient barrier against the penetration of substances in the urine. However, at the same time, this layer prevents the penetration of drugs instilled to the bladder. Also, the constant flow of urine inside the bladder reduces the therapeutic effect and washes the drug out of the organ. Thus, it becomes necessary to repeat this procedure several times. According to feedback from patients receiving medications through intravesical instillation, such manipulation is quite painful. At the same time, there is a high risk of infection and inflammation of the urethra if catheterization is performed incorrectly [3].
The development of drug carriers that are able to retain drugs at the urothelium surface of the bladder for a long time might overcome these limitations. In this regard, the development of micro- and nano-sized carriers for intravesical drug delivery is of great interest. The most prominent strategies described in the literature include various types of carriers including thermosensitive hydrogels capable of undergoing sol-gel transition at body temperature as well as site-specific targeted liposomes and nanoparticles. However, in the first case, the natural dilution of the gels in the bladder leads to the loss of their gel-forming properties [4,5]. In the second case, in order to obtain such micro-sized systems, synthetic hydrophobic polymers are used, which are often dissolved in organic solvents that are mostly non-biocompatible and have irritating effects on the tissues [6–8].
In our recent work, we developed biocompatible and biodegradable emulsion microgels based on whey isolate protein with sufficient mucoadhesive properties. Targeting of such micro-sized carriers into the bladder will extend the residence time of drugs, reducing the number of instillation procedures [9]. Thus, this approach will help to significantly improve the quality of life of patients suffering from diseases of the urinary system. However, it is necessary to carefully follow the protocol for the formation of emulsion microgels, since changing the ratios of precursors can lead to the formation of particles with different loading and mucoadhesive properties.
The protocol here presented describes in detail the procedure for the synthesis of fluorescently labeled emulsion microgels based on whey protein isolate. Calculations of the optimal oil-to-water ratios for obtaining the most stable emulsion systems are given. We consider the main ways to characterize the resulting particles and provide a step-by-step description of the methodologies and troubleshooting aspects involved in determining the loading capacity of emulsion microgels as well as their drug release rates. In addition, we provide precise guidance on how to perform qualitative and quantitative analysis of the mucoadhesive ability of microgels on porcine bladder tissue ex vivo.
Materials and reagents
Reagents
Linseed oil OLEOS (Russia) (unrefined cold pressed linseed oil, refractive index n20/D 1.4795, density 0.93 g/mL at 25 °C), https://oleos-info.ru/product/lnyanoe-maslo/
Whey protein isolate (WPI) California Gold Nutrition (USA), https://www.californiagoldnutrition.com/products/california-gold-nutrition-sport-whey-protein-isolate-unflavored-5-lb-2-27-kg-76479 (27 g protein, 6.2 g BCAAs, 4.7 g glutamic acid, low lactose. Lactose is used as an excipient, which is not involved in the formation of emulsion microgels. No additives or flavor enhancers)
Phosphate buffered saline (PBS) (Sigma-Aldrich, catalog number: P4417)
Tetramethylrhodamine isothiocyanate (TRITC) (Sigma-Aldrich, catalog number: T0820)
Fluorescein isothiocyanate isomer I (FITC) (Sigma-Aldrich, catalog number: F7250)
Rhodamine B isothiocyanate (RITC) (Sigma-Aldrich, catalog number: 283924)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P3911)
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C4901)
Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S6014)
Dimethyl sulfoxide (DMSO) (EcoChemAnalyt, Russia, CAS: 67-68-5)
Urea (CH4N2O) (ReaChem, Russia, State Standard 2081-92, CAS: 57-13-6)
Ammonium chloride (NH4Cl) (ReaChem, Russia, State Standard 3773-72, CAS: 12125-02-9)
Magnesium sulfate heptahydrate (MgSO4·7H2O) (ReaChem, Russia, State Standard 4523-77, CAS: 10034-99-8)
Sodium sulfate (Na2SO4) (ReaChem, Russia, State Standard 4166-76, CAS: 7757-82-6)
Sodium dihydrogen phosphate dihydrate (NaH2PO4·2H2O) (ReaChem, Russia, State Standard 245-76, CAS: 13472-35-0)
Sodium hydrogen phosphate (Na2HPO4) (ReaChem, Russia, State Standard 11773-76, CAS: 7558-79-4)
Hydrochloric acid (HCl) (EcoChemAnalyt, Russia, State Standard 14261-77, CAS: 7647-01-0)
Sodium hydroxide (NaOH) (EcoChemAnalyt, Russia, State Standard 4328-77, CAS: 1310-73-2)
Trypsin (Sigma-Aldrich, catalog number: T4799)
Milli-Q water (Merck Millipore, model: Milli-QTM Advantage A10TM, Germany)
Note: It is possible to use linseed oil from another manufacturer. However, it is worth paying attention to its properties (the refractive index n20/D of 1.4795 and the density of 0.93 g/mL at 25 °C).
Solutions
Saline 0.9% NaCl (w/v) solution (see Recipes)
2.5% WPI solution (see Recipes)
5% WPI solution (see Recipes)
7.5% WPI solution (see Recipes)
5 mg/mL FITC solution (see Recipes)
1 M NaOH solution (see Recipes)
PBS buffer (0.1 M, pH 8.3) (see Recipes)
Artificial urine solution (see Recipes)
0.5 mg/mL trypsin in Tris-HCl buffer solution (20 mL) (see Recipes)
70% ethanol (see Recipes)
Recipes
Note: In all recipes, liquid is added to the dry sample until the final specified volume is reached (“total” volume) in order to obtain an accurate solution concentration. For example, in Recipe 1, H2O is added to the dry sample of NaCl until the final volume of 1 L is reached (instead of just adding 1 L of H2O to the dry sample of NaCl). The volume of liquid is indicated in tables as a necessary recommended volume (not as a precise volume).
Saline 0.9% NaCl (w/v) solution
*w/v: weight (g) per volume (100 mL).
Reagent Final concentration Amount NaCl 0.9 % (w/v) 9 g H2O n/a 1 L Total n/a 1 L 2.5% WPI (w/v) aqueous solution
Reagent Final concentration Amount WPI 2.5 % 250 mg Saline 0.9% NaCl solution 0.9% (w/v) 10 mL Total n/a 10 mL Caution: This solution should be mixed thoroughly. Before use, please wait until the foam at the surface of the solution disappears.
Note: For the FITC conjugation procedure, WPI solution is prepared as described above in Recipe 2, but instead of water the PBS (pH 8.3) is used for protein dissolution.
5% WPI (w/v) aqueous solution
Reagent Final concentration Amount WPI 5% 500 mg Saline 0.9% NaCl solution 0.9% (w/v) 10 mL Total n/a 10 mL Caution: This solution should be mixed thoroughly. Before use, please wait until the foam at the surface of the solution disappears.
Note: For the FITC conjugation procedure, WPI solution is prepared as described above in Recipe 3, but instead of water the PBS (pH 8.3) is used for protein dissolution.
7.5% WPI (w/v) aqueous solution
Reagent Final concentration Amount WPI 7.5% 750 mg Saline 0.9% NaCl solution 0.9% (w/v) 10 mL Total n/a 10 mL Caution: This solution should be mixed thoroughly. Before use, please wait until the foam at the surface of the solution disappears.
Note: For the FITC conjugation procedure, WPI solution is prepared as described above in Recipe 4, but instead of water the PBS (pH 8.3) is used for protein dissolution.
5 mg/mL FITC solution
Reagent Final concentration Amount FITC 5 mg/mL 5 mg DMSO n/a 1 mL Total n/a 1 mL Note: Since a 5 mg sample is difficult to weigh, it is recommended to prepare a larger weight of FITC, which will require a larger volume of DMSO to prepare the final solution with the given FITC concentration of 5 mg/mL. For this, you need to proportionally increase FITC mass and DMSO volume. For example, weigh out 25 mg of FITC, then add DMSO until the volume reaches 5 mL. In this way, you will obtain 5 mL of a final FITC solution of 5 mg/mL.
1 M NaOH solution (100 mL)
Reagent Final concentration Amount NaOH 1 M 4 g H2O n/a 100 mL Total n/a 100 mL PBS solution (0.1 M, pH = 8.3)
Reagent Final concentration Amount PBS 1× 1 tablet H2O n/a 200 mL Total n/a 200 mL Note: Adjust pH of the resulting solution to 8.3 using 1 M NaOH and 1 M HCl solutions.
Artificial urine solution (pH 6.2)
Reagent Final concentration Amount Urea n/a 24.27 g NaCl n/a 6.34 g KCl n/a 4.50 g NH4Cl n/a 1.61 g CaCl2 n/a 0.67 g MgSO4·7H2O n/a 1.0 g NaHCO3 n/a 0.34 g Na2SO4 n/a 0.26 g NaH2PO4·H2O n/a 1.0 g Na2HPO4 n/a 0.11 g H2O n/a 2 L Total n/a 2 L Note: The dry weights and the liquid volume can be changed proportionally to each other depending on needs (for example, for 1 L, the masses of dry samples are correspondingly reduced by two times relative to the masses given in the table). It is recommended to add weights in the order of priority shown in the table. The solution should be stirred for 3 h at 22 °C. The prepared artificial urine solution should be stored at 2–8 .
0.5 mg/mL trypsin in Tris-HCl buffer solution (20 mL)
Reagent Final concentration Amount Trypsin 0.5 mg/mL 10 mg Tris base 1 M 2.42 g H2O n/a 20 mL Total n/a 20 mL Note: Adjust pH of the Tris solution to 7.5 using HCl 1 M. The solution should be stored at 2–8 °C.
Ethanol 70%
Reagent Final concentration Amount Ethanol 96% 70% 1 L H2O n/a 371 mL Total n/a 1,371 mL Note: When mixing these liquids, you must strictly add ethanol to water, not vice versa.
Laboratory supplies
Centrifuge tubes with flat cap, 15 mL (JetBioFil, catalog number: CFT550150)
Centrifuge tubes with flat cap, 50 mL (JetBioFil, catalog number: CFT500500)
Microcentrifuge tubes, 1.5 mL (JetBioFil, catalog number: CFT000015)
Microcentrifuge tubes, 2.0 mL (JetBioFil, catalog number: CFT000020)
Laboratory glass jar 100 mL, with divisions, with screw lid, dark glass (MiniMed, catalog number: 10007205)
Vial 2 mL, dark glass (ALWSCI Technologies, catalog number: C0001177)
Vial 10 mL, clear glass, 22 mm × 52 mm (ALWSCI Technologies, catalog number: C0000053)
Dialysis bag M-Cel, pore diameter 14 kDa (Viscase, catalog number: 2141-1425)
Dialysis bag clamp (Scienova GmbH, catalog number: 40329)
Laboratory beaker, clear glass, 2 L (MiniMed, catalog number:10003807)
Cellulose acetate membrane filter, 0.45 μm (Sartorius, catalog number: 11106-37-N)
Membrane filters PP, 10 μm (Gluvex, catalog number: MLPP1001000)
Glass for microslides (MiniMed, catalog number: 12003421)
Cover glass for microslides (MiniMed, catalog number: 12003309)
Syringe 1 mL (BD Micro-Fine Plus, catalog number: 320935)
96-well V-bottom plate, conical bottom, non-treated, no lid (Costar, catalog number: 3897)
Pipette microtips, 2–20 μL (JetBioFil, catalog number: PPT100020)
Pipette microtips, 10–200 μL (JetBioFil, catalog number: PPT000200)
Pipette microtips, 100–1,000 μL (JetBioFil, catalog number: PPT000000)
Petri dishes, D 9.0 cm (JetBioFil, catalog number: MCD000090)
Adson microsurgical tweezers, 130 mm (Medical Equipment, catalog number: MF-2000)
Tissue tweezers, 130 mm (Medical Equipment, catalog number: MF-2102)
Equipment
Ultrasonic homogenization and Bandelin Sonopuls HD 2070 homogenizer at a frequency of 20 kHz and a power density of 1 W/cm2 (Germany)
Amicon® Stirred Cell 50 mL (Merck Millipore)
Magnetic stirrer (IKA)
Magnetic stirring bar (IKA, model: IKAFLON® 15)
Multifunctional refrigerated centrifuge (Eppendorf, model: 5810R)
Microplate reader (BMG Labtech, model: CLARIO Star Plus)
Drybath thermo shaker (Thermo Scientific)
Inverted microscope with a 40× objective (Olympus IX73)
Water purification system for ultrapure water (Merck Millipore, model: Milli-QTM Advantage A10TM)
Single-channel variable volume dispenser 100–1,000 mL, 10–100 mL, 20–200 mL, 5–50 mL, and 1,000–5,000 mL (Thermo Fisher Scientific)
Software and datasets
ClarioStar MARS 4.01 R2 (BMG LabTech, Germany)
ImageJ software 1.51j8 (National Institutes of Health, USA) (the open-source version is available online for free for download)
OriginPro 2018 SR1 (OriginLab Corporation, USA)
Excel 2019 (Microsoft Cooperation, USA)
Procedure
文章信息
稿件历史记录
提交日期: Mar 21, 2024
接收日期: Jun 4, 2024
在线发布日期: Jun 24, 2024
出版日期: Jul 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Saveleva, M. S., Lobanov, M. E. and Mayorova, O. A. (2024). A Protocol for Preparing Mucoadhesive Emulsion Microgels and Assessing Their Mucoadhesion Properties In Vitro. Bio-protocol 14(13): e5027. DOI: 10.21769/BioProtoc.5027.
分类
生物工程 > 生物医学工程
医学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










