- EN - English
- CN - 中文
A Flow Cytometry–Based Method for Assessing CAR Cell Binding Kinetics Using Stable CAR Jurkat Cells
基于流式细胞术的稳定CAR-Jurkat细胞结合动力学评估方法
发布: 2024年06月20日第14卷第12期 DOI: 10.21769/BioProtoc.5021 浏览次数: 3160
评审: Alka MehraMartin V KolevNavnita DuttaAnonymous reviewer(s)
Abstract
Chimeric antigen receptors (CARs) are synthetic fusion proteins that can reprogram immune cells to target specific antigens. CAR-expressing T cells have emerged as an effective treatment method for hematological cancers; despite this success, the mechanisms and structural properties that govern CAR responses are not fully understood. Here, we provide a simple assay to assess cellular avidity using a standard flow cytometer. This assay measures the interaction kinetics of CAR-expressing T cells and targets antigen-expressing target cells. By co-culturing stably transfected CAR Jurkat cells with target positive and negative cells for short periods of time in a varying effector–target gradient, we were able to observe the formation of CAR-target cell doublets, providing a readout of actively bound cells. When using the optimized protocol reported here, we observed unique cellular binding curves that varied between CAR constructs with differing antigen binding domains. The cellular binding kinetics of unique CARs remained consistent, were dependent on specific target antigen expression, and required active biological signaling. While existing literature is not clear at this time whether higher or lower CAR cell binding is beneficial to CAR therapeutic activity, the application of this simplified protocol for assessing CAR binding could lead to a better understanding of the proximal signaling events that regulate CAR functionality.
Key features
• Determines CAR receptor cellular interaction kinetics using a Jurkat cell model.
• Can be used for a wide variety of CAR target antigens, including both hematological and solid tumor targets.
• Experiments can be performed in under two hours with no staining using a standard flow cytometer.
• Requires stable CAR Jurkat cells and target cells with stable fluorescent marker expression for optimal results.
Keywords: CAR-T (CAR-T)Graphical overview
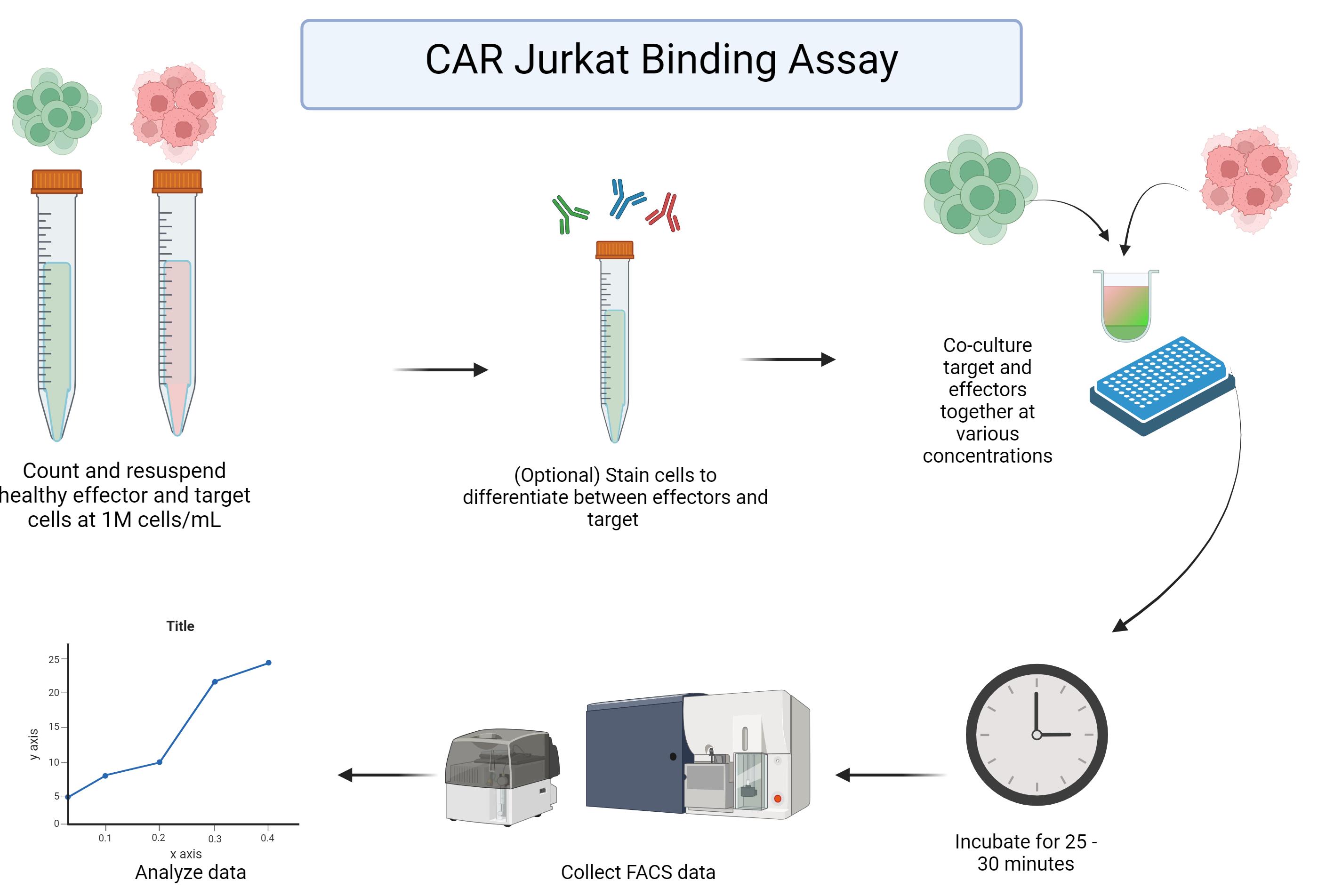
Background
Chimeric antigen receptor (CAR) T-cell therapy is an engineered cellular cancer therapy that reprograms a patient’s T cells to target a specific protein, mimicking natural T-cell receptor (TCR) function but redirected toward antigens found on the surface of cancer cells. CAR-T cells have emerged as a highly successful tool for providing robust therapeutic responses against refractory or relapsed hematological cancers, with ongoing research across many domains of immunology to extend the range and efficacy of CAR-T treatments for solid tumor malignancies [1–3].
Many advancements have been made in understanding the novel biology of CAR-modified T cells; however, CAR-T-cell screening models still struggle to choose candidate binding elements based on in vitro assessments of CAR-T activation and cytotoxicity assays. Whilst activation markers, such as CD69, are well-documented using CAR-Jurkat and primary CAR-T-cell screening methods, it is unclear how well these markers can predict in vivo efficacy of the CAR-T cells [4,5]. Recently, cellular avidity was recognized as a property of CAR-expressing cells that varies between different CAR constructs and correlates with important functional properties such as CAR expansion, CAR-activated trogocytosis, and CAR-T exhaustion [6–9].
Cellular avidity is defined by the total sum of cell-to-cell interactions, encompassing the whole immunological synapse and many cellular adhesion processes [10]. Given that cell binding is a near-immediate consequence of CAR signaling and proximal T-cell response processes, understanding the nature of this interaction and how it differs between standard TCR interaction vs. CARs is an important area of research. Investigating how cellular avidity changes with different CAR binding domains or structural designs is also vital for improving CAR discovery and development. It is important to note that while the specific properties of an antibody used to create a CAR impact the overall cellular avidity, the affinity and avidity of an antibody are entirely distinct from the complete cell-to-cell interaction that defines “cellular avidity”.
For soluble proteins such as antibodies, avidity is defined as the overall accumulated strength of interaction through multiple interactions, but it can vary widely depending on the nature of the target antigen and the specific conditions of the assay. The measured avidity of an antibody is usually reported as the half-maximal binding concentration observed over a titrated binding assay, this property being referred to as the apparent affinity (KD). In contrast to antibodies, using the assay described herein, we have observed that the half-maximal binding of CAR-expressing cells over a titrated range of target cell concentration does not vary between CAR proteins; rather, we find that CAR cells consistently reach a point of saturated maximal binding, wherein a varying proportion of CAR-expressing cells will form a strong bond with target cells (we refer to this as a CAR-target doublet). The strength of this interaction, while not encompassing the entirety of the kinetics, approximates what is commonly called cellular avidity. This observation of a varying binding rate between CAR-expressing and target cells has similarly been reported using an assay based on a resonance force ramp microscopy using the Lumicks zMovi device [6], which directly tests the strength of interaction by attempting to measure the force at which CAR-target pairs are dissociated. We find that the rate of CAR-target doublet formation increases with higher target cell density, eventually reaching a saturation point. Furthermore, we find the rate of doublet formation varies between different CARs [11] or CAR designs [5]. While this assay does not directly test the strength of interaction and therefore is only an approximation of overall cellular avidity, we believe the unique target binding properties for each CAR serve as valuable metrics for CAR screening and assessment. As such, this assay could be used as a replacement to, or in conjunction with, direct avidity assays for optimal CAR selection.
Here, we provide a complete description of our cellular binding assay that serves as a quick, high-throughput addition to CAR-T screening workflows using a standard flow cytometer. Through the quantification of cell/target doublets formed within CAR Jurkat target cell co-cultures over a wide range of effector-to-target ratios, the unique cell binding properties associated with each CAR can be determined. This assay provides consistent results and can be employed with both solid tumors (see validation of protocol) and hematological tumor models.
Materials and reagents
Biological materials
Jurkat e6.1 (ATCC, catalog number: TIB 152) expressing a variety of CAR proteins
Ramos (ATCC, catalog number: CRL-1596) or other relevant target tumor cell line(s)
Target cells have been engineered with Nuclight Lenti-Red (Sartorius, Germany; 4476). It is also possible to use non-fluorescent cells for this assay, but antibody prestaining of targets or effectors is recommended as detailed in section B.
Reagents
Fetal Bovine Serum (FBS) (Sigma Life Sciences, catalog number: F2442-500ML)
L-Glutamate (Gibco, catalog number: 25030-081)
Penicillin/Streptomycin (Pen/Strep) (Gibco, catalog number: 15140-122)
RPMI 1640 (Gibco, catalog number: 21870-076)
Dulbecco’s phosphate-buffered saline (DPBS) (Gibco, catalog number: 14190-144)
VHH or scFv-specific fluorescent antibody (optional) (generated in-house)
An analogous anti-VHH polyclonal product (Jackson Laboratories, catalog number: 128-605-232)
For other CARs, various anti-scFv or anti-linker antibodies are available
CD45 or other Jurkat/T-cell specific antibody (optional) (BD Pharmingen, catalog number: 560178)
CD19 or other target-specific antibody (optional) (BD Horizon, catalog number: 612938)
Solutions
R10 complete (see Recipes)
Recipes
R10 complete
Reagent Final concentration Quantity or Volume RPMI 1640 500 mL FBS 100 mL/L 50 mL L-Glutamate 10 mL/L 5 mL Pen/strep 10 mL/L 5 mL
Laboratory supplies
96-well U-bottom plate (Falcon, catalog number: 353077)
Equipment
BD LSRFortessa flow cytometer
BD LSRFortessa flow cytometer HTS plate reader
Software and datasets
FACS Diva
FlowJo
GraphPad Prism 10
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Shepherd, A., Bennychen, B., Ahmed, Z., Weeratna, R. D. and McComb, S. (2024). A Flow Cytometry–Based Method for Assessing CAR Cell Binding Kinetics Using Stable CAR Jurkat Cells. Bio-protocol 14(12): e5021. DOI: 10.21769/BioProtoc.5021.
分类
免疫学 > 免疫疗法 > CAR-T
细胞生物学 > 基于细胞的分析方法 > 细胞粘附
癌症生物学 > 肿瘤免疫学 > 癌症治疗
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link