- EN - English
- CN - 中文
Flow Cytometry Analysis of Microglial Phenotypes in the Murine Brain During Aging and Disease
衰老和疾病过程中小鼠大脑小胶质细胞表型的流式细胞术分析
发布: 2024年06月20日第14卷第12期 DOI: 10.21769/BioProtoc.5018 浏览次数: 3558
评审: Geoffrey C. Y. LauSuresh KumarAnonymous reviewer(s)

相关实验方案
外周血中细胞外囊泡的分离与分析方法:红细胞、内皮细胞及血小板来源的细胞外囊泡
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
2025年11月05日 1435 阅读
Abstract
Microglia, the brain's primary resident immune cell, exists in various phenotypic states depending on intrinsic and extrinsic signaling. Distinguishing between these phenotypes can offer valuable biological insights into neurodevelopmental and neurodegenerative processes. Recent advances in single-cell transcriptomic profiling have allowed for increased granularity and better separation of distinct microglial states. While techniques such as immunofluorescence and single-cell RNA sequencing (scRNA-seq) are available to differentiate microglial phenotypes and functions, these methods present notable limitations, including challenging quantification methods, high cost, and advanced analytical techniques. This protocol addresses these limitations by presenting an optimized cell preparation procedure that prevents ex vivo activation and a flow cytometry panel to distinguish four distinct microglial states from murine brain tissue. Following cell preparation, fluorescent antibodies were applied to label 1) homeostatic, 2) disease-associated (DAM), 3) interferon response (IRM), and 4) lipid-droplet accumulating (LDAM) microglia, based on gene markers identified in previous scRNA-seq studies. Stained cells were analyzed by flow cytometry to assess phenotypic distribution as a function of age and sex. A key advantage of this procedure is its adaptability, allowing the panel provided to be enhanced using additional markers with an appropriate cell analyzer (i.e., Cytek Aurora 5 laser spectral flow cytometer) and interrogating different brain regions or disease models. Additionally, this protocol does not require microglial cell sorting, resulting in a relatively quick and straightforward experiment. Ultimately, this protocol can compare the distribution of microglial phenotypic states between various experimental groups, such as disease state or age, with a lower cost and higher throughput than scRNA-seq.
Key features
• Analysis of microglial phenotypes from murine brain without the need for cell sorting, imaging, or scRNA-seq.
• This protocol can distinguish between homeostatic, disease-associated (DAM), lipid-droplet accumulating (LDAM), and interferon response (IRM) microglia from any murine brain region and/or disease model of interest.
• This protocol can be modified to incorporate additional markers of interest or dyes when using a cell analyzer capable of multiple color detections.
Keywords: Microglia (小胶质细胞)Graphical overview
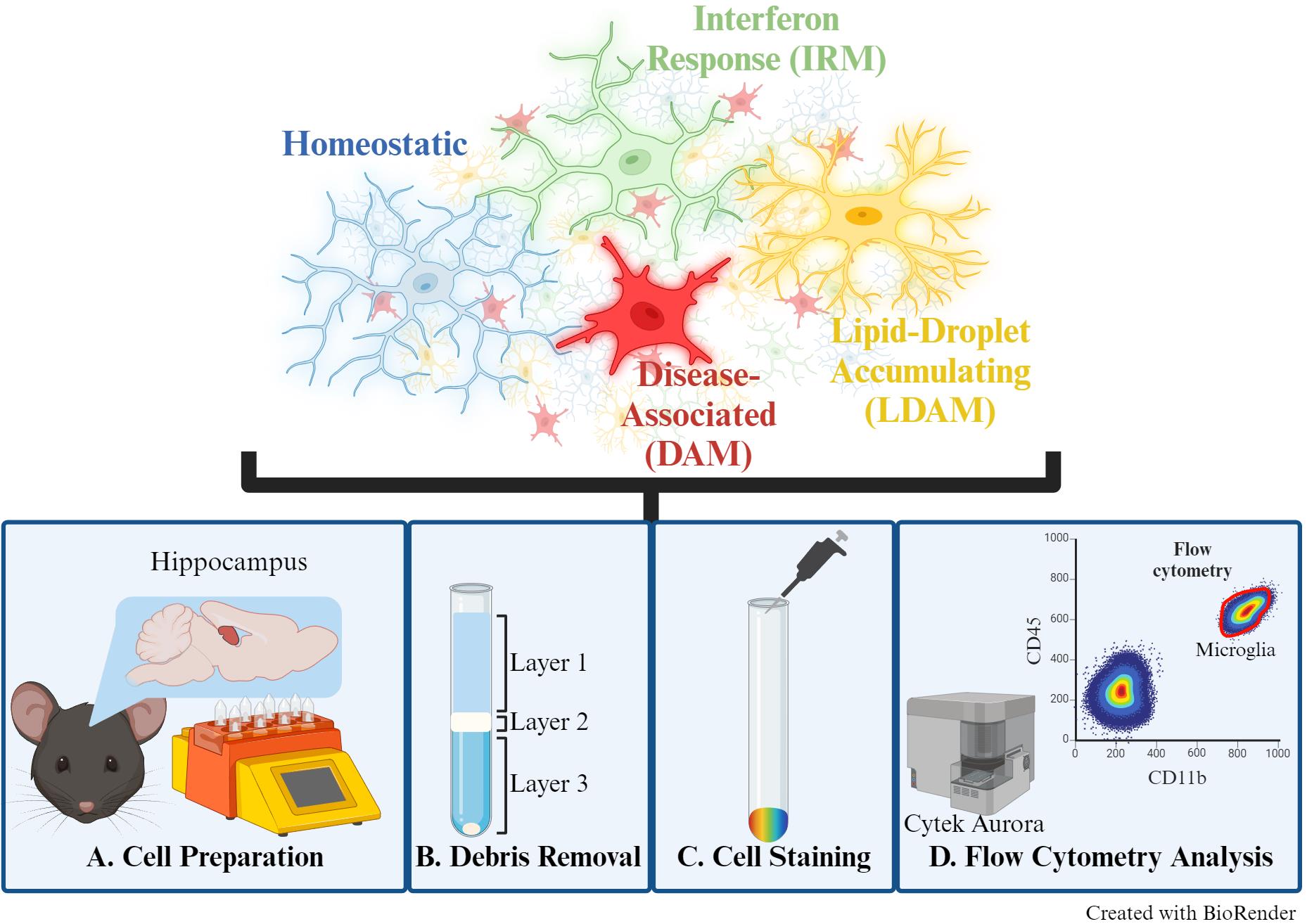
Background
Microglia are brain-resident immune cells that contribute to the neuroenvironment through diverse functions, including debris phagocytosis, neuronal support, and synaptic pruning. Their multifaceted roles align with distinct phenotypic states, largely influenced by environmental stimuli and intrinsic signaling cascades [1]. These specialized cells are involved in many neurological diseases, such as Alzheimer’s disease (AD). For instance, the disease-associated microglia (DAM) phenotype was first identified in an AD mouse model and has since been further validated to play a role in response to neurodegeneration [2]. Differentiating and quantifying microglial phenotypic states offers valuable insights into pathways associated with development and disease processes, presenting promising avenues for therapeutic exploration. Although fluorescent immunolabeling and microscopy can be used to distinguish microglial phenotypes, this approach is time-consuming, and quantification requires multiple tissue sections. Alternatively, sequencing-based approaches, though providing high-throughput and high-resolution data, are costly and demand specialized analytical training. Here, we present a fluorescence-based flow cytometry method to distinguish microglial phenotypic states, allowing for high-throughput, multiparametric analysis of single-cell suspensions with straightforward quantification tools.
In this protocol, we describe a simple and time-effective preparation of single-cell suspensions from murine brain tissue for flow cytometric analysis to distinguish multiple microglial phenotypes. Microglia have been previously described as either “M1” (pro-inflammatory) or “M2” (anti-inflammatory) to indicate “active” or “resting” states, respectively. However, the field has since moved to more descriptive terms such as “homeostatic” or specific “reactive” phenotypes, given that microglia are in a constant state of activity to respond to stimuli [3]. Therefore, this panel can distinguish between non-reactive/surveying (homeostatic) or reactive [DAM, interferon-response microglia (IRM), and lipid-droplet accumulating microglia (LDAM)] microglial phenotypes [3]. This protocol was previously used to examine sex differences in microglial phenotypic states during aging [4]. The markers for each of the examined phenotypes (homeostatic, DAM, IRM, and LDAM) were selected from several publications [2,5–10], ensuring no overlap between states, and are listed within the Materials and Reagents section.
A notable advantage to this protocol is the adaptability of this microglial phenotype panel, allowing for additional fluorescent antibodies or dyes depending on the laser and filter capabilities of the chosen cell analyzer. The use of fluorescent dyes offers in vivo examination of microglial phenotypes, including Methoxy-X04 (amyloid-β plaques) [11] and BODIPY (lipid accumulations) [10], where colocalization with these markers is useful in identifying DAM and LDAM phenotypes, respectively. This flexibility facilitates versatile applications in studying microglia. While initially utilized to examine sex differences in hippocampal microglial aging, this protocol can be applied to address various biological questions relevant to multiple murine disease models and other brain regions.
Materials and reagents
Biological materials
Mice: C57BL/6N (or any mouse line of interest)
Note: The data presented in the primary research article was generated in adult mice between 3 and 25 months of age. This protocol has not been tested on microglia isolated from neonatal or juvenile mice.
Reagents
Adult Brain Dissociation kit (Miltenyi Biotec, catalog number: 130-107-677)
D-PBS, calcium, magnesium, glucose, pyruvate (Gibco, catalog number: 14-287-072)
Cell staining buffer (BioLegend, catalog number: 420201)
TruStain FcX (BioLegend, catalog number: 101319)
Actinomycin D (Streptomyces species) (Sigma-Aldrich, catalog number: A1410-5MG)
Anisomycin (Streptomyces griseolus) (Sigma-Aldrich, catalog number: A9789-25MG)
Triptolide (Sigma-Aldrich, catalog number: T3652-1MG)
Dimethyl Sulfoxide (DMSO) (Fisher Scientific, catalog number: D1281)
Antibodies used for each microglia phenotype (Table 1)
Table 1. Antibody list for microglial phenotypes
Phenotype Antibody Conc. Fluorophore Clone Host Vendor Catalog # Dilution Canonical CD11b 0.2 mg/mL Brilliant Violet 421TM M1/70 Rat BioLegend 101235 1:50 Canonical (hematopoietic) CD45 0.2 mg/mL Brilliant Violet 785TM 30-F11 Rat BioLegend 103149 1:40 Homeostatic P2RY12 0.2 mg/mL APC/FireTM 810 S16007D Rat BioLegend 848013 1:50 Disease-associated microglia (DAM) CD11c 0.2 mg/mL Brilliant Violet 605TM N418 Arm. Hamster BioLegend 117334 1:50 Disease-associated microglia (DAM) CD282 0.2 mg/mL PE/Cyanine7 QA16A01 Mouse BioLegend 153011 1:50 Disease-associated microglia (DAM) CLEC7A 9 μg/300 μL APC REA154 Human Miltenyi 130102985 1:50 Interferon response Microglia (IRM) CD317 0.2 mg/mL Brilliant Violet 650TM 927 Rat BioLegend 127019 1:50 Lipid-droplet accumulating microglia (LDAM) CD63 0.2 mg/mL PE NVG-2 Rat BioLegend 143903 1:40
Solutions
Actinomycin D (5 mg/mL stock solution) (see Recipes)
Triptolide (10 mM stock solution) (see Recipes)
Anisomycin (10 mg/mL stock solution) (see Recipes)
Actinomycin D (5 mg/mL stock solution)
Reconstitute actinomycin D in DMSO to a concentration of 5 mg/mL.
Aliquot and store at -20 °C protected from light.
Triptolide (10 mM stock solution)
Reconstitute triptolide in DMSO to a concentration of 10 mM.
Aliquot and store at -20 °C protected from light.
Anisomycin (10 mg/mL stock solution)
Reconstitute anisomycin in DMSO to a concentration of 10 mg/mL.
Aliquot and store at 4 °C protected from light.
Laboratory supplies
GentleMACS C tubes (Miltenyi Biotec, catalog number: 130-096-334)
MACS SmartStrainer (70 μm) (Miltenyi Biotec, catalog number: 130-110-916)
15 mL centrifuge tubes (Corning, catalog number: 0553859A)
5 mL round-bottom Polystyrene test tube (Falcon, catalog number: 352054)
Strainer caps for FACS tubes (Olympus Plastics, catalog number: 28-155)
Equipment
GentleMACS Octo dissociator with heaters (Miltenyi Biotec, catalog number: 130-096-427)
Allegra X-30R centrifuge with swinging bucket (Beckman Coulter, catalog number: B08540)
Cytek Aurora 5 laser spectral flow cytometer (Cytek Biosciences, model: U0488, U1188)
Software and datasets
FlowJo v10.9.0
GraphPad Prism v9.5
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Cox, J. E. J., Pham, K. D., Keck, A. W., Wright, Z., Thomas, M. A., Freeman, W. M. and Ocañas, S. R. (2024). Flow Cytometry Analysis of Microglial Phenotypes in the Murine Brain During Aging and Disease. Bio-protocol 14(12): e5018. DOI: 10.21769/BioProtoc.5018.
分类
神经科学 > 细胞机理 > 小神经胶质
生物化学 > 蛋白质 > 标记
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link