- EN - English
- CN - 中文
Direct RNA Sequencing of Foot-and-mouth Disease Virus Genome Using a Flongle on MinION
使用MinION的Flongle直接测序口蹄疫病毒RNA基因组
发布: 2024年06月20日第14卷第12期 DOI: 10.21769/BioProtoc.5017 浏览次数: 2142
评审: Anthony SignoreMarcelo S. da SilvaAnonymous reviewer(s)

相关实验方案
诱导型HIV-1库削减检测(HIVRRA):用于评估外周血单个核细胞中HIV-1潜伏库清除策略毒性与效力的快速敏感方法
Jade Jansen [...] Neeltje A. Kootstra
2025年07月20日 2457 阅读
Abstract
Foot-and-mouth disease (FMD) is a severe and extremely contagious viral disease of cloven-hoofed domestic and wild animals, which leads to serious economic losses to the livestock industry globally. FMD is caused by the FMD virus (FMDV), a positive-strand RNA virus that belongs to the genus Aphthovirus, within the family Picornaviridae. Early detection and characterization of FMDV strains are key factors to control new outbreaks and prevent the spread of the disease. Here, we describe a direct RNA sequencing method using Oxford Nanopore Technology (ONT) Flongle flow cells on MinION Mk1C (or GridION) to characterize FMDV. This is a rapid, low cost, and easily deployed point of care (POC) method for a near real-time characterization of FMDV in endemic areas or outbreak investigation sites.
Key features
• Saves ~35 min of the original protocol time by omitting the reverse transcription step and lowers the costs of reagents and consumables.
• Replaces the GridION flow cell from the original protocol with the Flongle, which saves ~90% on the flow cell cost.
• Combines the NGS benchwork with a modified version of our African swine fever virus (ASFV) fast analysis pipeline to achieve FMDV characterization within minutes.
Keywords: Foot-and-mouth disease virus (口蹄疫病毒)Graphical overview
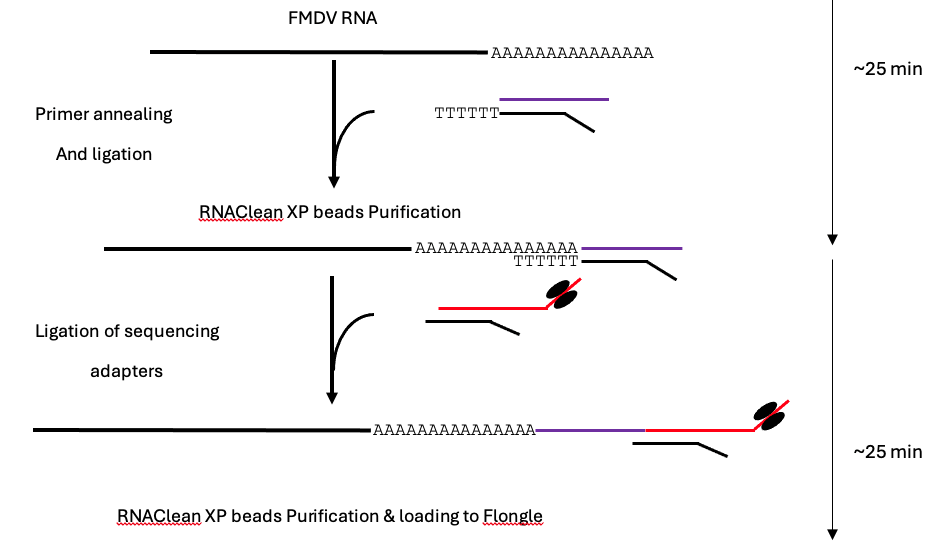
Schematic of direct RNA sequencing of foot-and-mouth disease virus (FMDV) process, which takes ~50 min from extracted RNA to final loading, modified from the ONT SQK-RNA002 protocol (Version: DRS_9080_v2_revO_14Aug2019).
Background
Foot-and-mouth disease (FMD) is a severe and extremely contagious viral disease that causes vesicular disease in cloven-hoofed domestic and wild animals. Outbreaks can lead to serious economic losses due to decreased livestock productivity and restrictions on movement and trade of animals and their products [1]. The causative agent of the disease is foot-and-mouth disease virus (FMDV), an Aphthovirus from the family Picornaviridae, with seven antigenically distinct serotypes including the EuroAsiatic serotypes A, O, C, and Asia 1, and the Southern African territories (SAT) serotypes 1, 2, and 3. Cross-protection is not conferred between serotypes following infection or vaccination and may not be conferred between different subtypes or variants of the same serotype [2]. The key factor to prevent the disease and control its spread is early detection and identification of the serotype(s) and subtype(s) of outbreak strains so that directed vaccination may be initiated. The antigenic determinants of FMDV reside within the structural proteins of the viral capsid (VP1-VP4), encoded by the genes 1A (VP4), 1B (VP2), 1C (VP3), and 1D (VP1). Historically, FMDV molecular epidemiology of outbreaks has focused on analyses of partial or full-length sequences of the 1D gene that encodes the structural protein VP1 [3]. Currently, over 60% (9571/14952 in December 2023) of FMDV records in the GenBank database have a total nucleic acid length of 400–700 bp and cover the partial or full 1D gene. Unfortunately, relying solely on the VP1 coding sequence does not provide all of the information required to fully characterize important phenotypic traits of FMDV strains, since several antigenic determinants are located on other viral capsid proteins, such as VP2 and VP3 [4,5]. Moreover, during shorter epidemic time scales, sequencing of 1D cannot provide enough resolution to discriminate the viruses collected from adjacent premises within the same outbreak clusters due to the viral populations not having diverged substantially [6]. In this regard, numerous reports have been published using the whole P1 region, encoding all structural proteins (i.e., VP1 to VP4) for strain genotyping and phylogenetic analysis [7,8]. The characterization of the whole P1 region (1A to 1D) is important for tracking the emergence or spread of FMD and for selecting vaccines in case of an outbreak. However, restricting analyses to P1 sequences may not detect recombination events that can occur between multiple serotypes or topotypes in the event of co-circulating FMDV within infected animals [5,9,10]. Next-generation sequencing (NGS) techniques offer much promise to perform viral whole genome sequencing (WGS) without prior knowledge about the target sequence and provide a huge amount of data from a limited quantity of starting material in a rapid, cost-effective manner. Valdazo-González and colleagues (2012)[11] have demonstrated that WGS of FMDV is a powerful tool for the reconstruction of transmission trees.
WGS of FMDV has been previously conducted on different platforms including Oxford Nanopore Technology (ONT) and Illumina [6,10,12–14]. These methods use reverse transcriptase to convert RNA to cDNA and/or go through one or two FMDV-specific or non-specific PCR amplifications. Some are labor-intensive with cumbersome procedures; others may have relatively longer turnaround times. Moreover, viral RNA manipulation can introduce biases into the data [15], which may not represent the actual diversity within samples [6]. Here, we introduce for the first time an FMDV direct RNA sequencing method using Nanopore sequencing on Flongle flow cells with MinION Mk1C (or GridION). Compared to the original ONT SQK-RNA002 protocol, we omitted the optional reverse transcription step to make the process even shorter by saving ~35 minutes as the ONT device only sequences the RNA molecule, not the cDNA. Our method is low-cost, fast, and reliable and can be deployed to low-resource settings when using an Mk1C device, such as a remote or field laboratory with alternative RNA extraction methods. This POC method requires less than 50 min of manipulation time from RNA to NGS run (Graphical Abstract), followed by a rapid identification of the whole FMDV genome within minutes.
Materials and reagents
Biological materials
Seven FMDV isolates were used to develop the RNA direct sequencing method, one of each serotype (see Table 4 in the Evaluation of Protocol section below). The isolates were obtained from the Biorepository in the Reagents and Vaccine Services Section of FADDL as viral stocks propagated from cell lines identified in Table 4.
Reagents
MagMAXTM CORE Nucleic Acid Purification kit (Thermo Fisher, catalog number: A32702 or A32700)
Qubit RNA High Sensitivity (HS) Assay kit (Thermo Fisher, catalog number: Q32852 or Q32855)
Ethyl alcohol, molecular grade (e.g., Thermo Fisher, catalog number: T032021000)
RNAClean XP beads (Beckman Coulter, catalog number: A63987)
Direct RNA Sequencing kit (ONT, catalog number: SQK-RNA002)
Flow Cell Priming kit (ONT, catalog number: EXP-FLP001)
Flongle sequencing expansion (ONT, catalog number: EXP-FSE001)
NEBNext® quick ligation reaction buffer (New England Biolabs, catalog number: B6058)
T4 DNA ligase 2,000,000 units/mL (New England Biolabs, catalog number: M0202)
Flongle flow cell (ONT, catalog number: FLO-FLG001)
Calibrated pipettes single (P1000, P200, P100, P20, P10, P2) (e.g., Rainin)
Pipette tips, aerosol resistant, RNase free (P1000, P1000X, P200, P200X, P20) (Rainin, catalog numbers: 30389212, 30389323, 0389239, 30389242, 30389225)
1.5 mL DNA LoBind® tubes (Eppendorf, catalog number: 022431021)
Qubit tubes (Thermo Fisher, catalog number: Q32856)
Nuclease-free water (Thermo Fisher, catalog number: AM9937)
Racks for 1.5 mL tubes
Magnetic stands (e.g., Thermo Fisher, catalog number: 12321D)
Timers (e.g., Cole-Parmer, catalog number: EW-90225-35)
Ice or ice packs (e.g., Fisher Scientific, Corning Ice Pan Mini 1L, catalog number: Corning 432116)
PPE: Disposable lab coats, nitrile gloves, eye goggles
Equipment
KingFisherTM Duo Prime purification system (Thermo Fisher, catalog number: 5400110)
Qubit 4 fluorometer (Thermo Fisher, catalog number: Q33238)
Scilogex SCI-M analog microplate mixer (Scilogex, catalog number: 822000049999)
Scilogex Mixer accessories: universal circular adapter (Scilogex, catalog number: 18900067)
Scilogex Mixer accessories: foam test tube insert for 12 tubes (Scilogex, catalog number: 18900022)
Microcentrifuges to hold 1.5–2 mL tubes and 0.2 mL PCR tubes
MinION Mk1C (Oxford Nanopore Technologies)
GridION (Oxford Nanopore Technologies)
Flongle adapter (ONT, catalog number: ADP-FLG001)
Cold storage devices: Refrigerator (+4 °C) for flow cells and some reagents, freezer (-20 °C) for enzymes and other reagents, ultra-low freezer (-70 °C) for storage of RNA samples
Software and datasets
MinION Mk1C (or GridION) devices with up to date MinKNOW software
The host genome FASTA files were downloaded from GenBank (Table 1), uploaded to MinION Mk1C (or GridION), and then used as references on the adaptive sampling step of the MinKNOW runtime setting to deplete the host sequence reads
Table 1. Host genome used as references on the adaptive sampling step of MinKNOW
Species Genome version used Bovine Bovine UMD3.1_chromosomes Swine GCF_000003025.6_Sscrofa11.1_genomic Guinea pig GCF_000151735.1_Cavpor3.0_guineaPig Sheep Ovis aries (sheep) GCA_016772045.1_ARS-UI_Ramb_v2.0
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Xu, L., Berninger, A., Lakin, S. M., O’Donnell, V., Pierce, J. L., Pauszek, S. J., Barrette, R. W. and Faburay, B. (2024). Direct RNA Sequencing of Foot-and-mouth Disease Virus Genome Using a Flongle on MinION. Bio-protocol 14(12): e5017. DOI: 10.21769/BioProtoc.5017.
分类
微生物学 > 病原体检测
微生物学 > 微生物-宿主相互作用 > 病毒
分子生物学 > RNA > RNA 检测
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link