- EN - English
- CN - 中文
Transfection of Babesia duncani: A Genetic Toolbox of This Pathogen to Advance Babesia Biology
邓氏巴贝斯虫基因转染:推进巴贝斯虫生物学的遗传工具箱
发布: 2024年06月20日第14卷第12期 DOI: 10.21769/BioProtoc.5016 浏览次数: 2057
评审: Alba BlesaRITU SOMAnonymous reviewer(s)
Abstract
Human babesiosis is a tick-borne disease caused by Babesia pathogens. The disease, which presents with malaria-like symptoms, can be life-threatening, especially in individuals with weakened immune systems and the elderly. The worldwide prevalence of human babesiosis has been gradually rising, prompting alarm among public health experts. In other pathogens, genetic techniques have proven to be valuable tools for conducting functional studies to understand the importance of specific genes in development and pathogenesis as well as to validate novel cellular targets for drug discovery. Genetic manipulation methods have been established for several non-human Babesia and Theileria species and, more recently, have begun to be developed for human Babesia parasites. We have previously reported the development of a method for genetic manipulation of the human pathogen Babesia duncani. This method is based on positive selection using the hDHFR gene as a selectable marker, whose expression is regulated by the ef-1aB promoter, along with homology regions that facilitate integration into the gene of interest through homologous recombination. Herein, we provide a detailed description of the steps needed to implement this strategy in B. duncani to study gene function. It is anticipated that the implementation of this method will significantly improve our understanding of babesiosis and facilitate the development of novel and more effective therapeutic strategies for the treatment of human babesiosis.
Keywords: Babesiosis (巴贝虫病)Graphical overview
Background
Babesia are parasitic protozoa of the Apicomplexa phylum, which encompasses other important human pathogens such as the agents of malaria and toxoplasmosis. Babesia parasites are transmitted primarily by ticks and can infect various mammalian species [2]. In humans, babesiosis is caused by several Babesia species including B. microti, B. duncani, B. divergens, and B. venatorum [3]. Clinical manifestations of the disease include fever, hemolytic anemia, and in severe cases, complications such as cardiac failure, respiratory distress, and pulmonary issues, which can ultimately lead to death. Patients with underlying immunological disorders, undergoing immunosuppressive treatment, or having undergone splenectomy are particularly susceptible to experiencing severe symptoms and increased mortality if infected by Babesia [3–5]. B. duncani is found primarily in western United States and exhibits higher virulence in animal models compared to B. microti, leading to acute mortality in mice and in hamsters [6–9]. Morphologically, there are no significant distinguishing features between B. duncani and B. microti [10].
Gene editing has become an invaluable tool for the investigation of gene function and validation of potential drug targets [11]. Advancing our understanding of the fundamental biology of Babesia parasites requires the development of genetic manipulation techniques [12]. Previous studies have demonstrated successful genetic manipulation methods in various protozoan pathogens, including Babesia bovis, B. gibsoni, B. ovis, B. ovata, Theileria annulata, and Theileria parva [13–18]. Successful transfection of piroplasmids was achieved in B. bovis [1] using the elongation factor 1-aB (ef-1aB) promoter to drive expression of a reporter GFP-BSD consisting of a fusion between the green fluorescent protein (GFP) and the blasticidin S deaminase (BSD) [19], which was integrated into the ef-1αA region through homologous recombination. Subsequent studies employed a similar approach for stable transfection in B. gibsoni, B. ovata, and Babesia sp. Xinjiang [17,18,20]. While a genetic modification method has been reported for B. microti, it mainly relies on fluorescent tags due to the lack of effective in vivo drug screening markers, challenging the generation of gene-edited strains in B. microti [21]. Genetic manipulation of human Babesia parasites has also been facilitated by the availability of a continuous in vitro culture system in human red blood cells and the sequencing, assembly, and annotation of their genomes [5,9,22–24]. Unlike B. microti, for which only a short-term culture could be achieved, long-term and continuous in vitro culture of B. duncani has been established using hamster and human erythrocytes [24–27]. Furthermore, in B. duncani, an animal model of lethal infection has been optimized, thus paving the path for evaluating the importance of specific genes in vivo [8,27]. The first genetic modification of B. duncani [28] was achieved using a transient transfection technique to express the mCherry reporter in the parasite under the regulatory control of the ef-1αB promoter. Subsequently, a stable expression of eGFP was achieved in B. duncani using the human dihydrofolate reductase (hDHFR) gene selectable marker, which confers resistance to the antifolate WR99210. The development of genetic tools for the manipulation of B. duncani provides a unique opportunity to study gene function in B. duncani and to gain further insights into its biology and pathogenesis.
Development of the protocol
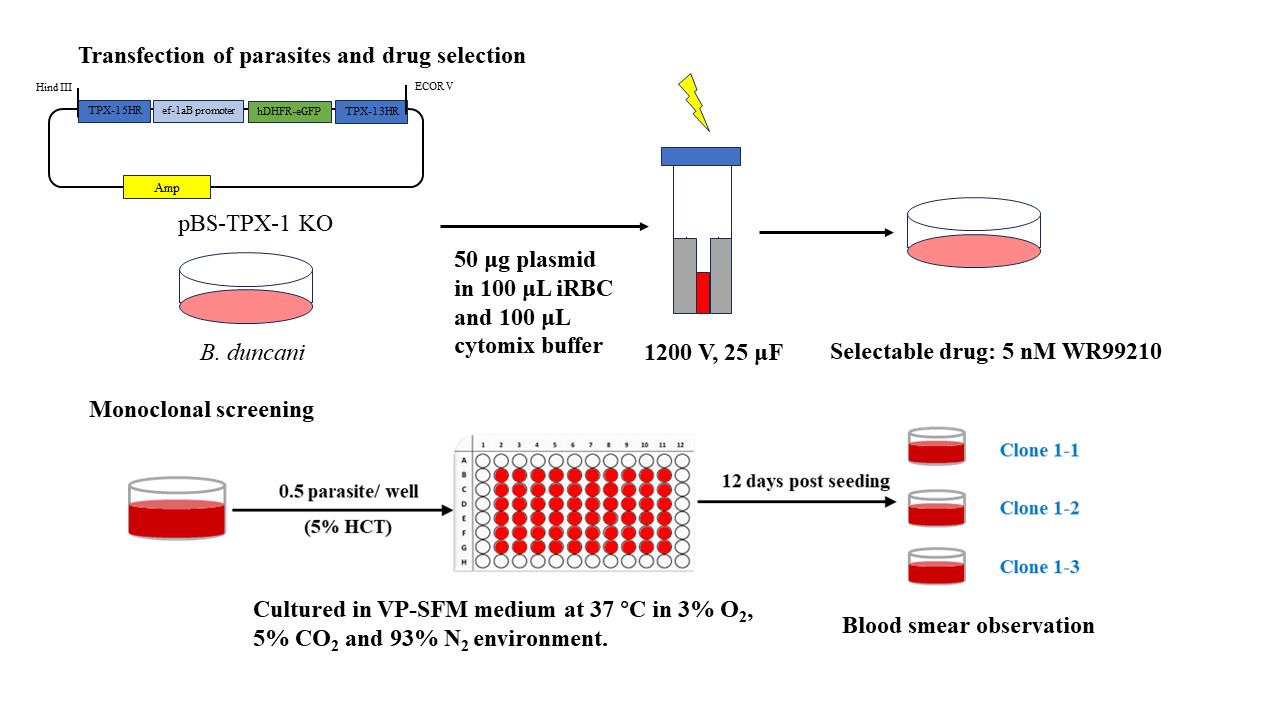
The electroporation method has been widely utilized for various apicomplexan parasites, including Toxoplasma gondii (the causative agent of toxoplasmosis) and Plasmodium spp. (the agents of human malaria). Electroporation is an effective means of introducing foreign DNA into these parasites for genetic manipulation and functional studies. Electroporation methods are indeed categorized into two main types based on the electrical parameters. The first is low voltage–long pulse, which employs a lower electrical voltage for a relatively longer pulse duration. This approach is typically considered gentler and is often used when cell viability is a primary concern. The high voltage–short pulse method involves using a high electrical voltage for a very short pulse duration. It is often referred to as the burst or high-field electroporation. This approach is more efficient in terms of introducing foreign material into cells but may have a greater impact on cell viability. The choice between these methods depends on the specific requirements of the experiment, such as the type of cells or organisms being electroporated and the desired level of transfection efficiency vs. cell survival. In B. duncani, as in previously reported cases in B. bovis [1], the high voltage–short pulse electroporation method results in higher transfection efficiency. To enhance transfection efficiency in B. duncani, our optimization efforts focused on changing the parameters of electroporation, including voltage, volume, and frequency, with the aim of achieving higher levels of transfection efficacy.
When comparing different electroporation instruments, we usually find variations in their effectiveness for transfection. We found that a higher transfection efficiency could be achieved using the Gemini SC Electroporation System from BTX, whereas transfection using the Bio-Rad Gene Pulser Xcell was not successful.
Materials and reagents
Biological materials
Chemically competent E. coli DH5α [F− supE44 ΔlacU169 (φ80 lacZDM15) hsdR17 (rk−mk) recA1 endA1 thi1 gyrA relA] is used for routine cloning and plasmid maintenance. The competent cells are available via ThermoFisher (catalog number: 18265017)
Hamster RBCs are used for B. duncani culture. The hamster RBCs are obtained from Syrian golden hamsters [Crl: LVG (SYR)] and can be prepared following the protocol.
B. duncani WA1 strain (ATCC PRA-302™) is expanded through in vitro cultivation and the protocol.
MilliQ water
Bacto tryptone (Becton Dickinson, catalog number: 211705)
Bacto yeast extract (Becton Dickinson, catalog number: 212750)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S5886)
Bacto agar (Becton Dickinson, catalog number: 214010)
Ampicillin sodium salt (Sigma-Aldrich, catalog number: A9518)
Phanta Max Super-Fidelity DNA Polymerase (Vazyme, catalog number: P505)
2× Rapid Taq Master Mix (Vazyme, catalog number: P222)
ClonExpress MultiS One Step Cloning Kit (Vazyme, catalog number: C113)
100 bp DNA ladder (Vazyme, catalog number: MD104-01)
EasyPure® Quick Gel Extraction kit (Trans, catalog number: EG-101)
EasyPure® Plasmid MiniPrep kit (Trans, catalog number: EM-101)
EndoFree Maxi plasmid kit (TIANGEN, catalog number: DP117)
TIANamp Geneomin DNA kit (TIANGEN, catalog number: DP304)
VP-SFM ATGTM (Thermo Fisher Scientific, Gibco, catalog number: 12559019)
Ala-Gln (MACKLIN, catalog number: A800584)
AlbuMax II (Thermo Fisher Scientific, Gibco, catalog number: 11021037)
Antimycotic (antibiotic) (Thermo Fisher Scientific, Gibco, catalog number: 15240-062)
WR99210 (MCE, catalog number: HY-116387)
MBP146-78 (TargetMol, catalog number: T7321)
Giemsa’s stain (Sigma-Aldrich, catalog number: G4507)
Immersion oil (Sigma-Aldrich, catalog number: 1046990100)
Trisodium citrate (Sigma-Aldrich, catalog number: V900443)
Citric acid (Sigma-Aldrich, catalog number: C0759)
Glucose (Sigma-Aldrich, catalog number: G8270)
Sodium dihydrogen phosphate (Sigma-Aldrich, catalog number: 5438400100)
Adenine (Sigma-Aldrich, catalog number: A2786)
Mannitol (Sigma-Aldrich, catalog number: M4125)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: S5886)
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C5670)
Dipotassium hydrogen phosphate (K2HPO4) (Sigma-Aldrich, catalog number: P8281)
Potassium dihydrogen phosphate (KH2PO4) (Sigma-Aldrich, catalog number: P5655)
4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES) (Sigma-Aldrich, catalog number:H4034)
Ethylene glycol-bis(2-aminoethylether)-N, N, N', N'-tetraacetic acid (EGTA) (Sigma-Aldrich, catalog number: E3889)
Magnesium chloride hexahydrate (MgCl2·6H2O) (Sigma-Aldrich, catalog number: M2393)
Saponin (Sigma-Aldrich, catalog number: S7900)
Absolute ethanol (Sigma-Aldrich, catalog number: 1009831011)
Alsever’s solution (Sigma-Aldrich, catalog number: A3551)
DMSO (Sigma-Aldrich, catalog number: D8418)
Glycerol (Sigma-Aldrich, catalog number: G5516)
Tris (MACKLIN, catalog number: T819512)
Ethylenediaminetetraacetic acid (EDTA) (MACKLIN, catalog number: E809068)
Acetic acid (MACKLIN, catalog number: A801295)
Potassium hydroxide (KOH, MACKLIN, catalog number: P816399)
Agarose (MACKLIN, catalog number: A800342)
GelRed (MACKLIN, catalog number: G917739)
Ethidium bromide (EB, MACKLIN, catalog number: E808961)
Sodium acetate buffer, 3 M, pH 5.2 (MACKLIN, catalog number: S885174)
Hoechst (Beyotime, catalog number: CH1024)
Solutions
LB medium (see Recipes)
LB plates (see Recipes)
Ampicillin stock solutions (100 mg/mL) (see Recipes)
TAE buffer (see Recipes)
VP-SFM complete media (see Recipes)
Red blood cell preservation solution (see Recipes)
Cytomix buffer (see Recipes)
20% Saponin stock solution (see Recipes)
5 mM WR99210 storage solution (see Recipes)
Freezing solution (see Recipes)
Recipes
LB medium
Prepare LB medium by dissolving the following ingredients (with the help of a magnetic stirrer) in MilliQ water: 10 g/L tryptone, 5 g/L yeast extract, and 5 g/L NaCl.
Aliquot the broth in clean bottles, autoclave at 121 °C for 15 min, and let it cool down before use.
LB medium can be stored at room temperature for up to six months.
LB plates
Prepare the LB medium as described above, aliquot in clean bottles, and add 15 g/L Bacto agar. Autoclave at 121 °C for 15 min and let cool down to ~50 °C at room temperature before the addition of antibiotics or other supplements (when needed). Mix well and pour ~20 mL into Petri dishes. Plates can be stored at 4 °C for up to one month.
Ampicillin stock solutions (100 mg/mL)
Weigh 1,000 mg of ampicillin sodium salt using an electronic scale and dissolve in 10 mL of MilliQ water. Filter the solution through a 0.2 μm filter in the laminar flow hood and prepare 1 mL aliquots in sterilized 1.5 mL microcentrifuge tubes. Antibiotic stock solutions can be stored at -20 °C for up to one year.
TAE buffer
The 50× TAE stock solution contains 2 mol/L Tris (242 g/L), 0.1 mol/L EDTA disodium salt (37.2 g/L), and 57.1 mL/L acetic acid. Add double-distilled water (ddH2O) to a final volume of 1 L, place the solution on the magnetic stirrer, and stir until it is completely dissolved. Once dissolved, adjust the pH to 8.0. The 50× TAE stock solution can be stored at room temperature for several years; dilute it with ddH2O to 1× TAE buffer before use.
VP-SFM complete media
Prepare VP-SFM complete medium by dissolving the following ingredients (with the help of a magnetic stirrer) in MilliQ water: 17.6 g/L VP-SFM ATGTM, 0.86 g/L Ala-Gln, 2 g/L AlbuMax II, and 10 mL/L 100× antibiotic/antimycotic. Filter the solution through a 0.2 μm filter in the laminar flow hood and prepare 50 mL aliquots in a sterilized centrifuge tube. Medium can be stored at 4 for up to six months.
Red blood cell preservation solution
Prepare red blood cell preservation solution by dissolving the following ingredients (with the help of a magnetic stirrer) in MilliQ water: 1.5 g/L trisodium citrate, 0.2 g/L citric acid, 7.93 g/L glucose, 0.94 g/L sodium dihydrogen phosphate, 0.14 g/L adenine, 4.97 g/L sodium chloride, and 14.57 g/L mannitol. Aliquot the solution in clean bottles, autoclave at 121 °C for 15 min, and let it cool down before use. Red blood cell preservation solution can be stored at 4 for up to six months.
Cytomix buffer
In a clean and sterile container or bottle, add approximately 800 mL of MilliQ water. Carefully add the dry ingredients in the following order while gently stirring to ensure proper dissolution: 6.7 g KCl, 0.012 g CaCl2, 1.30 g K2HPO4, 1.02 g KH2PO4, 4.46 g HEPES, 0.76 g EGTA, and 0.762 g MgCl2·6H2O; adjust the pH 7.5 with potassium hydroxide (KOH). After all the components are dissolved, carefully adjust the volume to 1 L using MilliQ water and mix thoroughly to ensure the solution is homogenous. Filter the solution through a 0.2 μm filter in the laminar flow hood and prepare 50 mL aliquots in a sterilized centrifuge tube. Medium can be stored at 4 for up to six months.
20% Saponin stock solution
Weigh 20 g of saponin using an electronic scale and dissolve in 100 mL of 1× PBS (8.0 g/L NaCl, 0.2 g/L KCl, 1.44 g/L Na2HPO4, 0.24 g/L KH2PO4, PH 7.4). Filter the solution through a 0.2 μm filter in the laminar flow hood and prepare 1 mL aliquots in sterilized 1.5 mL microcentrifuge tubes. Saponin stock solutions can be stored at −20 °C for up to one year. Dilute the saponin solution to 0.1% with PBS when lysing red blood cells.
5 mM WR99210 storage solution
Add 0.5067 mL of DMSO to 1 mg of WR99210 powder. Gently swirl to dissolve. Prepare small 10 μL aliquots and store at -20 °C for up to 12 months. Prepare a 5 μM working concentration by diluting it in DMSO at a 1:1,000 ratio before use.
Freezing solution
In a 50 mL centrifuge tube, add 15 mL of glycerol, followed by 35 mL of Alsever's solution. Mix the solution by vortex thoroughly and then filter it using a 0.2 μm filter. Freezing solution can be stored at 4 °C for up to six months.
Laboratory supplies
Micropipette refill tips (10, 200, and 1,000 µL)
1.5 mL Eppendorf tube (Pullen, catalog number: PL03001)
PCR tubes, 8 tubes per strip, 125 strips per unit (Crystalgen (GY), catalog number: L-2081)
PCR tubes, domed 8 caps per strip, 125 strips per unit (Crystalgen (GY), catalog number: L-2082)
24-well plates (Corning, catalog number: 3524)
96-well plates (Corning, catalog number: 3917)
15 mL centrifuge tube (PULLEN, catalog number: PL01005)
50 mL centrifuge tube (PULLEN, catalog number: PL01006)
1 mL syringe (Beyotime, catalog number: FS802)
Equipment
Micropipette set, Eppendorf Research plus (0.1–2.5, 2–20, 20–200, and 100–1,000 µL) (Eppendorf, catalog number: 3123000012, 3123000098, 3123000055, and 3123000063)
Laboratory glass bottles (250, 500, and 1,000 mL)
T100 thermal cycler (Bio-Rad, catalog number: 1861096)
Petri dishes (Ø × H: 92 × 16 mm, with ventilation cams)
Benchtop centrifuge (Eppendorf, catalog number: 5810R)
Electric thermostatic incubator (Shanghai Jinghong Instrument, catalog number: DNP-9272)
Floored large refrigerated shaking incubator (Shanghai Zhichu Instrument, catalog number: ZQLY-300S)
Medical icebox (Panasonic, catalog number: MPR-710)
Low temperature freezer (-20 °C) (Panasonic, catalog number: MDF-539)
Gemini SC electroporation system (BTX, catalog number: 45-2042)
pH meter (Sartorius, model: PB-11)
Gene Pulser/MicroPulser electroporation cuvettes, 0.2 cm gap (Bio-Rad, catalog number: 1652082)
Modular incubator chamber (Billups-Rothenberg, Inc, model: MIC-101)
Millipore 0.2 μM filters (Sigma-Aldrich, catalog number: SLGNDZ5)
Blue-light gel cutter (Sangon, model: G600312-000)
NanoDrop 2000 (Thermo Fisher, model: pedestal mode)
Water bath (Beyotime, model: E0530)
Gel imaging system (Bio-Rad, model: XXX)
OLYMPUS FRAME_BX63 scanning confocal microscope (OLYMPUS FRAME, model: BX63)
Cell counter (Watson, model: 177-112C)
Nalgene Mr. Frosty Freezing Container (Thermo Scientific, catalog number: 5100-0036)
Software
SnapGene (RRID: SCR_015052, https://www.snapgene.com, SnapGene 7.0.2)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Wang, S., Wang, J., Li, D., Chen, F., Luo, W., Zhao, J. and He, L. (2024). Transfection of Babesia duncani: A Genetic Toolbox of This Pathogen to Advance Babesia Biology. Bio-protocol 14(12): e5016. DOI: 10.21769/BioProtoc.5016.
分类
微生物学 > 微生物遗传学 > 基因组编辑
分子生物学 > DNA > 转染
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link