- EN - English
- CN - 中文
Fast, Easy, and Comprehensive Techniques for Microscopic Observations of Fungal and Oomycete Organisms Inside the Roots of Herbaceous and Woody Plants
快速、简便且全面的草本和木本植物根内真菌和卵菌显微观察技术
发布: 2024年06月05日第14卷第11期 DOI: 10.21769/BioProtoc.5013 浏览次数: 2119
评审: Lucy XieAnonymous reviewer(s)
Abstract
The roots of herbaceous and woody plants growing in soil are complex structures that are affected by both natural and artificial fungal colonization to various extents. To obtain comprehensive information about the overall distribution of fungi or oomycetes inside a plant root system, rapid, effective, and reliable screening methods are required. To observe both fine roots, i.e., a common site for penetration of fungi and oomycetes, and mature roots, different techniques are required to overcome visual barriers, such as root browning or tissue thickening. In our protocol, we propose using fast, cost-effective, and non-harmful methods to localize fungal or oomycete structures inside plant roots. Root staining with a fluorescent dye provides a quick initial indication of the presence of fungal structures on the root surfaces. The protocol is followed by clearing and staining steps, resulting in a deeper insight into the root tissue positioning, abundance, and characteristic morphological/reproductive features of fungal or oomycete organisms. If required, the stained samples can be prepared by using freeze-drying for further observations, including advanced microscopic techniques.
Key features
• The protocol enhances tissue-clearing techniques employing KOH or NaOH and is applicable to a broad range of roots from different plant species.
• Hydroxides are mixed with hydrogen peroxide to obtain an efficient bleaching solution, which effectively clears roots without causing significant tissue damage.
• The protocol could also be used for staining of fungi or oomycetes localized both on the root surface or inside the root tissues.
• Simple combination of non-fluorescent methyl blue and fluorescent solophenyl flavine dyes allows the observation of fungal organisms in both brightfield and fluorescence microscopy.
Keywords: Fungi staining (真菌染色)Graphical overview
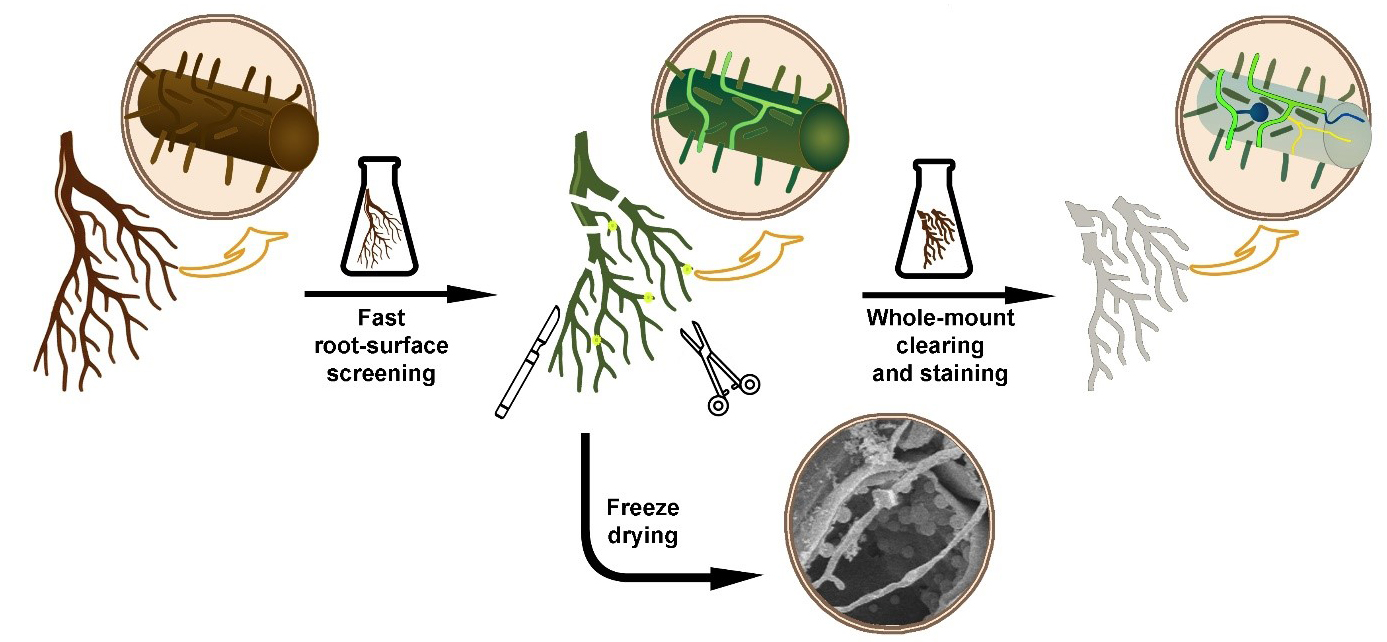
Background
Fungal and oomycete organisms, regardless of whether they are pathogenic or symbiotic, enter the roots of herbaceous or woody plants and grow inside their tissues. However, the minimal optical transparency of such roots is a key factor limiting direct microscopic observations of colonizing fungal organisms inside their root environment. Thin and relatively transparent young roots still do not allow efficient microscopic observation of their internal structures, including inside growing fungal organisms. In addition, older roots are much thicker and accumulate a lot of colorants, which deteriorates the observation at a microscopic level. Finally, when looking for fungi inside the root, they must be stained to contrast with the root tissues. To overcome these problems, diverse plant microtechniques allow different ways to visualize and effectively locate the fungal organism inside root tissues, for example, whole-mount root clearing and subsequent staining of fungal organisms [1], root hand sectioning followed by clearing and staining [2], or marking the fungi with fluorescent GFP [3]. The effectiveness of such techniques is limited by the time needed for their realization. Processing large root systems of herbs and especially woody plants requires a lot of time to find and visualize the fungal organisms in them. The main aim of this protocol is to combine the above-mentioned techniques of fluorescent marking and whole-mount clearing to offer a fast and easy way to observe the fungal and oomycete organisms inside the root tissues.
A key feature that enables staining at the root surface is the fact that fluorescent dye solophenyl flavine 7GFE 500 (syn. Direct Yellow 96) provides a fluorescent signal from the fungal structures but not from the root cells. Solophenyl flavine was introduced by Hoch et al. [4] as a new dye applicable for fungal cell-wall staining. Like calcofluor-white, a cellulose-specific fluorescent dye, solophenyl flavine also stains fungal structures. Moreover, the excitation and emission wavelengths of solophenyl flavine are shifted toward the red end of the spectrum, so the excitation is less dependent on UV or violet light. Moreover, the bright yellow–green signal from solophenyl flavine–stained fungi strongly contrasts against the background of a dark root. This makes screening effective for a quick inspection of the root surface. If deeper inspection of the root is required, clearing by removing cell protoplasm makes root tissues transparent. This is normally performed by using chemicals like NaOH or KOH [5]. If dark colorants still persist inside the root tissues, they need to be bleached before staining [6]. Hydrogen peroxide can bleach the roots and make them white. In this protocol, we use a mixture of NaOH (or KOH) with hydrogen peroxide as an efficient clearing/bleaching solution [7]. Metals that could be released from the samples, leading to reduced activity of the hydrogen peroxide, are chelated following the addition of sodium citrate. As hydrogen peroxide is extremely reactive, which results in bubble release, magnesium sulfate is added to prevent tissue damage caused by the air bubbles [8]. A key feature that allows staining inside the root is a mild clearing procedure that protects the root tissue structures and the fungal structures inside the root. Clearing also protects cell-wall binding sites, important for both cell wall–dye interaction and effective staining. Roots cleared this way can be stained again using solophenyl flavine. In some cases, however, there is a need for brightfield staining of fungal organisms. Dyes commonly used for non-fluorescent fungal staining, such as trypan blue [9], chlorazol black [10], or lactophenol cotton blue [11], are effective but harmful substances. In this protocol we use modified lactophenol cotton blue solution without phenol, which is harmful. Instead, methyl blue is mixed with glycerol and lactic acid to obtain blue staining of fungal organisms. If required, the protocol also offers a possibility of mixing methyl blue with solophenyl flavine and staining for both brightfield and fluorescence microscopy in one step. The proposed protocol improves the existing root-clearing techniques by using a shorter time and a lower clearing temperature. This improvement is achieved by combining clearing and bleaching into a single step and delicate sample handling.
Materials and reagents
Biological materials
Alnus glutinosa, Oryza sativa, or Arabidopsis thaliana roots or any other root material
Reagents
Potassium hydroxide (KOH) (Centralchem, CAS: 1310-58-3)
Sodium hydroxide (NaOH) (Centralchem, CAS: 1310-73-2)
Hydrogen peroxide, 50% (H2O2) (Centralchem, CAS: 7722-84-1)
Sodium citrate dihydrate [HOC(COONa)(CH2COONa)2·2H2O] (Sklochem-Agroekolab, CAS: 6132-04-3)
Magnesium sulphate heptahydrate (MgSO4·7H2O) (Sigma-Aldrich, CAS: 10034-99-8)
Methyl blue (C37H27N3Na2O9S3) (Fisher Chemical, CAS: 28983-56-4) (please do not confuse with methylene blue)
Glycerol [HOCH2CH(OH)CH2OH] (Duchefa Biochemie, CAS: 56-81-5)
Lactic acid, 80% [CH3CH(OH)COOH] (Mikrochem, CAS: 50-21-5)
Solophenyl flavine 7GFE 500 (syn. Direct Yellow 96) (C39H34N10O13S4) (abcr Germany, CAS: 61725-08-4)
1% HCl
Solutions
1% NaOH bleaching solution—sample clearing and bleaching (see Recipes)
10% KOH bleaching solution—sample clearing and bleaching (see Recipes)
0.06% methyl blue—staining solution (stock) (see Recipes)
0.006% methyl blue—non-fluorescent fungi staining (working solution)
0.1% solophenyl flavine—fluorescent fungi staining (see Recipes)
Methyl blue + solophenyl flavine mixture—combined fluorescent and non-fluorescent fungi staining (see Recipes)
Recipes
1% NaOH bleaching solution (BS)
To prepare 100 mL of 1% NaOH BS, first dissolve 0.9 g of NaOH in 90 mL of distilled water. Add 1 g of sodium citrate dihydrate and 0.03 g of magnesium sulphate heptahydrate into 90 mL of 1% NaOH. Using a stirrer to dissolve all components. A large volume of prepared solution may be stored for months in a dark place. Mix thoroughly before using.
Prior to clearing, add 10 mL of 50% H2O2 into 90 mL of the prepared NaOH + sodium citrate dihydrate + magnesium sulphate heptahydrate solution. Stir shortly to minimize hydrogen peroxide reaction but be sure to mix the prepared solution well.
Reagent Final concentration Quantity or Volume NaOH 1% (w/v) 0.9 g Sodium citrate dihydrate 1% (w/v) 1 g Magnesium sulphate heptahydrate 0.03% (w/v) 0.03 g Hydrogen peroxide 5% (v/v) 10 mL H2O 90 mL Total 100 mL 10% KOH bleaching solution (BS)
Alternatively, for 10% potassium hydroxide bleaching solution (KOH BS), dissolve 9 g of KOH in 90 mL of distilled water. Other chemicals and steps are applied in the same order as for Recipe 1.
Reagent Final concentration Quantity or Volume KOH 10% (w/v) 9 g Sodium citrate dihydrate 1% (w/v) 1 g Magnesium sulphate heptahydrate 0.03% (w/v) 0.03 g Hydrogen peroxide 5% (v/v) 10 mL H2O 90 mL Total 100 mL Although hydrogen peroxide alone can bleach, mixing NaOH or KOH with hydrogen peroxide shortens the time required for clearing. Individual clearing with NaOH or KOH followed by hydrogen peroxide will work too, but mixed compounds work faster.
0.06% methyl blue stock solution
To make 0.06% methyl blue stock solution, dissolve methyl blue in water using a stirrer. Then, mix glycerol with lactic acid. Finally, add methyl blue solution into glycerol/lactic acid mixture and stir well. This solution can be stored in a cool, dark place for months. Before staining, prepare 0.006% working solution by diluting 5 mL of methyl blue stock solution in 50 mL of distilled water.
Reagent Final concentration Quantity or Volume Methyl blue 0.06% (w/v) 0.03 g Glycerol 50% (v/v) 25 mL Lactic acid 80% 25% (v/v) 12.5 mL H2O 12.5 mL Total 50 mL 0.1% solophenyl flavine staining solution
To prepare a 0.1% solophenyl flavine staining solution, dissolve 0.025 g of solophenyl flavine powder in 25 mL of distilled water and mix thoroughly. This solution can be stored in a cool, dark place for one month.
Reagent Final concentration Quantity or Volume Solophenyl flavine 0.1% (w/v) 0.025 g H2O 25 mL Total 25 mL Methyl blue + solophenyl flavine mixture
To obtain 50 mL of methyl blue + solophenyl flavine mixture, add 1 mL of 0.1% solophenyl flavine into 49 mL of 0.006% methyl blue working solution. Stir well.
Laboratory supplies
Petri dishes of various size (it depends on the size of the examined root system) for root processing and staining:
Petri dish Ø150 mm (Fisher Slovakia, catalog number: 1215.1525)
Petri dish Ø120 mm (Fisher Slovakia, catalog number: 1215.1220)
Petri dish Ø50 mm (Fisher Slovakia, catalog number: 1215.0512)
Petri dish Ø40 mm (Fisher Slovakia, catalog number: 1215.0412)
Crystallizing dish 500 mL (Fisher Slovakia, catalog number: 1212.0115)
Beaker 250 mL (Fisher Slovakia, catalog number: 1112.0250)
Beaker 150 mL (Fisher Slovakia, catalog number: 1112.0150)
Beaker 50 mL (Fisher Slovakia, catalog number: 1112.0050)
Glass slide 76 mm × 26 mm (Fisher Slovakia, catalog number: 1820.1100)
Cover glass 24 mm × 50 mm (Fisher Slovakia, catalog number: 1820.1250)
Parafilm M 50 mm × 50 mm (Fisher Slovakia, catalog number: 2105.6001)
Razor blade (Agar Scientific, catalog number: AGT5115)
Laboratory scissors (Fisher Slovakia, catalog number: 2305.7711)
Laboratory needle (Fisher Slovakia, catalog number: 2305.4822)
Laboratory tweezer (Fisher Slovakia, catalog number: 2305.4143)
Scalpel (Fisher Slovakia, catalog number: 2305.9004)
Disposable plastic Pasteur pipette (Fisher Slovakia, catalog number: 2101.6460)
Pasteur glass pipette (Fisher Slovakia, catalog number: 1780.0150)
Magnetic stir bar (Fisher Slovakia, catalog number: 6115.4008)
Micropipette 1,000 µL (Fisher Slovakia, catalog number: 4052.0050)
Flat paint brush
Equipment
Heating plate with regulated temperature (30–70 °C)
Light (brightfield) and fluorescent microscope (Leica, model: DM4000) equipped with a Leica fluorescence filter A, D, I3 or any other filter with the following characteristics: excitation 400 nm and emission 470 nm
Freeze dryer (Christ Alpha 1-2 LD plus) (or any other freeze-drying machine), if freeze-drying is considered to be carried out
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Toma, T., Kováč, J. and Ďurkovič, J. (2024). Fast, Easy, and Comprehensive Techniques for Microscopic Observations of Fungal and Oomycete Organisms Inside the Roots of Herbaceous and Woody Plants. Bio-protocol 14(11): e5013. DOI: 10.21769/BioProtoc.5013.
分类
植物科学 > 植物免疫 > 宿主-细菌相互作用
植物科学 > 植物细胞生物学 > 细胞染色
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link