- EN - English
- CN - 中文
A New Approach for Assessment of Neutrophil Extracellular Traps Through Immunofluorescence Staining in Whole Blood Smears
通过免疫荧光染色在全血涂片中评估中性粒细胞胞外网的新方法
发布: 2024年06月05日第14卷第11期 DOI: 10.21769/BioProtoc.5010 浏览次数: 2840
评审: Pilar Villacampa AlcubierreSaskia F. Erttmann
Abstract
Neutrophils, constituting 50%–70% of circulating leukocytes, play crucial roles in host defense and exhibit anti-tumorigenic properties. An elevated peripheral blood neutrophil-to-lymphocyte ratio is associated with decreased survival rates in cancer patients. In response to exposure to various antigens, neutrophils release neutrophil granular proteins, which combine to form web-like structures known as neutrophil extracellular traps (NETs). Previously, the relative percentage of NETs was found to be increased in resected tumor tissue samples from patients with gastrointestinal malignancies. The presence of NETs in peripheral blood is indicative of underlying pathological conditions. Hence, employing a non-invasive method to detect NETs in peripheral blood, along with other diagnostic tests, shows potential as a valuable tool not just for identifying different inflammatory disorders but also for assessing disease severity and determining patient suitability for surgical resection. While reliable methods exist for identifying NETs in tissue, accurately quantifying them in whole blood remains challenging. Many previous methods are time-consuming and rely on a limited set of markers that are inadequate for fully characterizing NETs. Therefore, we established a unique sensitive smear immunofluorescence assay based on blood smears to identify NETs in only as little as 2 μL of whole blood. To identify the NET complexes that have enhanced specificities, this combines the use of various antibodies against neutrophil-specific CD15, NET-specific myeloperoxidase (MPO), citrullinated histone H3 (Cit H3), and nuclear DNA. This protocol offers an easy, affordable, rapid, and non-invasive method for identifying NETs; thus, it can be utilized as a diagnostic marker and targeted through various therapeutic approaches for treating human malignancies.
Key features
• Characterization of neutrophil extracellular traps in whole blood smears through immunofluorescence staining.
• Affordable and quantitative approach to neutrophil extracellular trap detection.
Keywords: NETosis (NETosis)Graphical overview
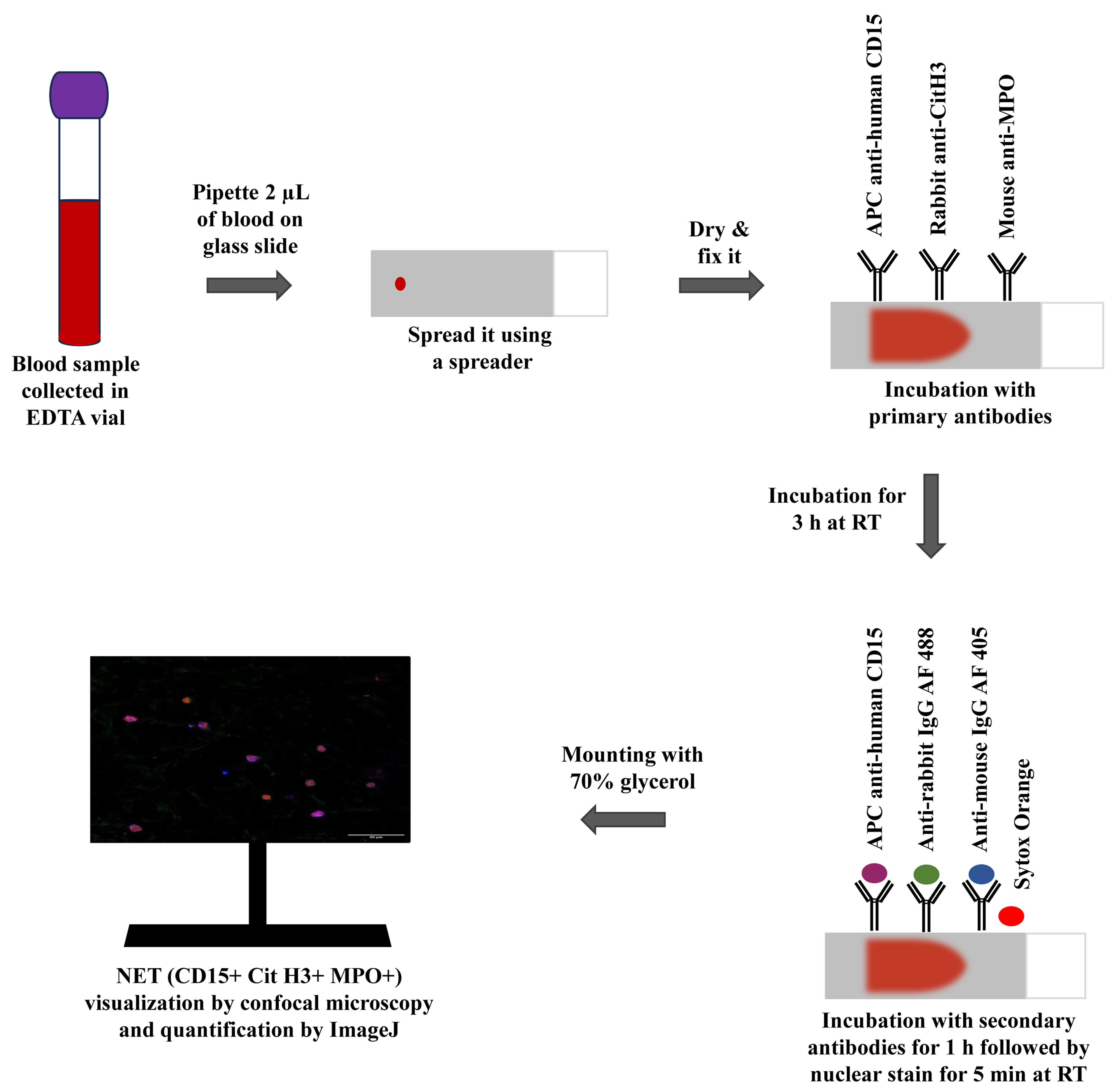
Graphical representation of the immunofluorescence-based blood smear assay to characterize neutrophil extracellular traps.
Background
Cancer is a serious global public health issue, and an upsurge in advanced-stage disease and mortality may result from delays in diagnosis and treatment [1]. Pancreatic ductal adenocarcinoma (PDAC), the most prevalent histologic variant of pancreatic cancer (PC), is considered one of the most aggressive solid tumors, having a high metastatic potential with a 5-year survival rate of less than 5% [2]. According to estimates, PDAC will overtake the principal cause of cancer mortality by 2030, ranking third globally in terms of cancer-associated fatalities [2,3]. It is currently widely acknowledged that immunological dysfunction and carcinogenesis are intimately related [4]. The most prevalent type of immune cell, neutrophils, comprising approximately 50%–70% of all circulating leukocytes, are essential for both the inflammatory and immune responses to solid tumors [5]. Neutrophils are responsible for maintaining the immune system of the host and provide not only the defense against foreign antigens but also anti-tumorigenic properties. The behavior of neutrophils can vary depending on the various stimuli: N1 neutrophils show pro-inflammatory and anti-tumorigenic properties, while N2 neutrophils show anti-inflammatory and pro-tumorigenic properties. According to recent research, a high peripheral blood neutrophil-to-lymphocyte ratio is associated with a poorer probability of survival for cancer patients [6]. Neutrophils, upon activation by cytokines or a variety of stimuli in the tumor microenvironment, undergo a cellular response called neutrophil extracellular trap (NET) formation (NETosis), in which DNA, along with histone and granule proteins, are extruded into the tissue surroundings and ultimately come into circulation [7]. A crucial enzyme in the production of NETs is peptidyl arginine deiminase 4 (PADI4), which catalyzes the deamination of arginine on histone H3 to induce chromatin decondensation and subsequent DNA extrusion [8]. The proteins that constitute NETs vary depending on the stimulus; nevertheless, the NET core signature proteins are histones, neutrophil elastase (NE), and myeloperoxidase (MPO), which are present regardless of the stimulus [9].
NETosis is a physiological phenomenon in which NETs are formed from neutrophils. Physiologically, NETs were found to be involved in host immune defense against fungi, parasites, and viruses [7]. However, studies on a variety of malignancies have revealed that NETs are crucial to the development of tumors [8]. Previously, the relative percentage of NETs was found to be increased in resected tumor tissue samples from patients with gastrointestinal malignancies [10,11]. Through the promotion of the epithelial-to-mesenchymal transition (EMT), NETs enhanced the ability of cancer cells to migrate and invade [12]. According to recent research, NETs trap circulating tumor cells, which promotes the growth and metastasis of the primary tumor, being directly correlated with the burden of metastatic disease [13,14]. Through the trapping of cancer cells and the reawakening of dormant cells through ECM remodeling, NETs may conspire to promote cancer recurrence [15]. NETs have also been observed to aid in the advancement of PC patients' tumor angiogenesis, venous thrombosis, and inhibition of apoptosis [16,17]. Overall, these findings indicate that NET detection could be significant for cancer diagnosis and prognosis.
Clinical evidence suggests that tumor-associated neutrophils (TANs) correlate with poor prognosis, and the tumor microenvironment plays a crucial role in controlling neutrophil recruitment. However, the extent of infiltration of neutrophils and their differentiation into NETs in the tumor microenvironment in a variety of cancers including PDAC remains unexplored [9]. Therefore, the need of the hour is to gain insight into the efficient characterization of NETs, so that their role in tumor progression can be deciphered. The purpose of this work was to identify NETs in the peripheral blood of PDAC patients. Detecting NETs in peripheral blood may indicate underlying pathological conditions. Thus, using a non-invasive approach to identify NETs, alongside other diagnostics, holds promise for identifying inflammatory disorders, assessing disease severity, and determining suitability for surgical resection. To that end, a unique smear immunofluorescence assay was established, which can identify NETs in only as little as 2 μL of blood. This study could aid in developing a diagnostic biomarker for various diseases and a therapeutic approach to boost immunotherapy following curative cancer excision.
Materials and reagents
APC anti-human CD15 (SSEA-1) (BioLegend, catalog number: 323008)
Mouse monoclonal antibody [2C7] to myeloperoxidase (MPO) (Abcam, catalog number: 25989)
Rabbit polyclonal antibody to histone H3 (anti-Cit H3 citrulline R2 + R8 + R17) (Abcam, catalog number: 5103)
Goat polyclonal antibody to rabbit IgG Alexa Fluor 488 (2 mg/mL) (Abcam, catalog number: ab150077)
Goat polyclonal antibody to mouse IgG Alexa Fluor 405 (2 mg/mL) (Abcam, catalog number: ab175660)
Sytox Orange nucleic acid stain, 5 mM solution in DMSO (Invitrogen, catalog number: S11368)
10× phosphate buffered saline (PBS), pH 7.2 (HiMedia, catalog number: TL1032-500mL)
Tween 20 (Sigma, catalog number: 9005-64-5)
Triton X-100 (HiMedia, catalog number: MB031-500mL)
Bovine serum albumin (BSA) (HiMedia, catalog number: MB083-100g)
Paraformaldehyde (PFA) (Nice Chemicals, catalog number: P64929)
Glycerol (≥ 99.5%) (HiMedia, catalog number: MB060)
Ultra-pure distilled water
Solutions
Wash buffer (see Recipes)
Fixing buffer (see Recipes)
Dilution buffer (see Recipes)
Permeabilization buffer (see Recipes)
Blocking buffer (see Recipes)
70% glycerol (see Recipes)
Recipes
Wash buffer (1× PBS)
Reagent Final concentration Quantity or Volume 10× PBS 1× PBS 5 mL Distilled water ~ 45 mL HCl 1 M Mix 5 mL of 10× PBS with 40 mL of distilled water. Adjust to pH 7.2 with 1 M HCl. Adjust the final volume up to 50 mL with distilled water. Store wash buffer at 4 °C.
Fixing buffer
Reagent Final concentration Quantity or Volume PFA 4% 10 g 1× PBS NA ~250 mL NaOH 1 M Dissolve 10 g of PFA in 200 mL of 1× PBS in a heating and stirring block (60 °C) and cool down upon complete dissolving of PFA. Add 1 M NaOH solution (4 g of NaOH pellets dissolved in 100 mL of distilled water) dropwise to adjust to pH 6.9. Adjust the final volume up to 250 mL with 1× PBS. Store aliquots at 4 °C (see General note 1).
Dilution buffer
Reagent Final concentration Quantity or Volume BSA 1% 0.5 g 1× PBS NA 50 mL Gently mix the solution by inverting the reaction tube. Sterile filter with 0.22 µm pore microfilter. Store at 4 °C.
Permeabilization buffer
Reagent Final concentration Quantity or Volume 1× PBS NA 100 mL Tween 20 0.1% 100 µL Triton X-100 0.5% 500 µL Prepare this solution in the dark and mix well. Store at 4 °C in air-tight amber bottles, as Tween 20 and Triton X-100 are light-sensitive.
Blocking buffer
Reagent Final concentration Quantity or Volume BSA 5% 0.5 g 1× PBS NA 10 mL Gently mix the solution by inverting the reaction tube. Sterile filter with 0.22 µm pore microfilter. Store at 4 °C.
70% glycerol
Reagent Final concentration Quantity or Volume Glycerol 70% 35 mL 1× PBS NA 15 mL Prepare 70% glycerol solution by mixing 35 mL of glycerol with 15 mL of 1× PBS. Use pH paper to ensure a pH of ~7.4. Acidic glycerol will cause rapid fading of fluorochromes. Store at 4 °C in air-tight amber bottles, as glycerol is light sensitive.
Laboratory supplies
15 mL conical centrifuge tubes (Tarsons, catalog number: 546021)
50 mL conical centrifuge tubes (Tarsons, catalog number: 546041)
BD Vacutainer K2EDTA vials (BD, catalog number: 454020)
BD EmeraldTM single-use syringe, 5 mL (BD, catalog number: 307725)
LifeLongTM 24 G needle, 0.55 mm × 25 mm (Lifelong, catalog number: 021824-H)
Microscope slides, 75 mm long × 25 mm wide, thickness 1.35 mm (Blue Star, catalog number: PIC-1)
Microscopic cover glass, rectangular (Special) 22 mm × 60 mm (Blue Star, catalog number: 5128789)
PierceTM microcentrifuge tubes, 1.5 mL (Thermo Scientific, catalog number: 69715)
PierceTM microcentrifuge tubes, 2.0 mL (Thermo Scientific, catalog number: 69720)
Syringe-driven filters, filter pore size 0.22 µm, diameter 30 mm (HiMedia, catalog number: SF137-100NO)
Advanced PAP Pen, 5 mm tip width (Sigma, catalog number: Z377821)
Borosilicate glass graduated round reagent bottles with screw caps (Borosil, catalog number: 1519)
Graduated cylinder (Borosil, catalog number: 3021)
Humidifier slide chamber (we used customized humidifier slide chamber of dimensions 35 cm × 30 cm × 6 cm from Seven Star Scientific Instruments. Researchers can also use Evergreen Scientific slide moisture chamber of dimensions 81/4×7×11/4" from VWR, catalog number: 76278-832) (see General note 4)
Qualigens Labolene Neutral pH 5 L (Thermo Fisher Scientific, catalog number: Q42218)
Sterile pipette tips 0.5–10 µL, 1–200 µL, 100–1,000 µL volume range (Tarsons)
Equipment
Micro-pipettes 0.5–10 µL, 10–100 µL, 20–200 µL, 100–1,000 µL (Eppendorf)
Class II biological safety cabinet (ESCO Lifesciences, model: AC2-4S8-NS)
SPINOTTM Digital Magnetic Stirrer Hot Plate (Tarsons, model: MC 02, Catalogue number: 6040)
Olympus Fluoview confocal laser scanning microscope (Olympus, model: FV3000)
RO-DI Ultra (Rions Labpure water solutions, model: ASTM Type I)
Software and datasets
ImageJ; Java-based program (1.5.4) (https://imagej.nih.gov/ij/download.html)
Microsoft Office Professional Plus 2019
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Bansal, S., Sharma, V., Gupta, R., Singh, H. and Aggarwal, A. (2024). A New Approach for Assessment of Neutrophil Extracellular Traps Through Immunofluorescence Staining in Whole Blood Smears. Bio-protocol 14(11): e5010. DOI: 10.21769/BioProtoc.5010.
分类
癌症生物学 > 肿瘤免疫学 > 免疫学试验
免疫学 > 免疫细胞染色 > 免疫检测
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link