- EN - English
- CN - 中文
A Multi-Color Immunofluorescence Assay to Distinguish Intracellular From External Leishmania Parasites
一种多色免疫荧光检测法区分细胞内外利什曼原虫
发布: 2024年06月05日第14卷第11期 DOI: 10.21769/BioProtoc.5009 浏览次数: 1870
评审: Chiara AmbrogioRamu KakumanuAnonymous reviewer(s)
Abstract
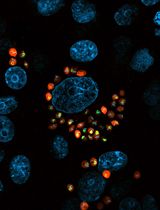
Leishmaniasis, a neglected tropical disease, is caused by the intracellular protozoan parasite Leishmania. Upon its transmission through a sandfly bite, Leishmania binds and enters host phagocytic cells, ultimately resulting in a cutaneous or visceral form of the disease. The limited therapeutics available for leishmaniasis, in combination with this parasite’s techniques to evade the host immune system, call for exploring various methods to target this infection. To this end, our laboratory has been characterizing how Leishmania is internalized by phagocytic cells through the activation of multiple host cell signaling pathways. This protocol, which we use routinely for our experiments, delineates how to infect mammalian macrophages with either promastigote or amastigote forms of the Leishmania parasite. Subsequently, the number of intracellular parasites, external parasites, and macrophages can be quantified using immunofluorescence microscopy and semi-automated analysis protocols. Studying the pathways that underlie Leishmania uptake by phagocytes will not only improve our understanding of these host–pathogen interactions but may also provide a foundation for discovering additional treatments for leishmaniasis.
Key features
• This protocol visualizes and quantifies multiple intracellular forms of Leishmania.
• It offers flexibility at various points for researchers to introduce modifications according to their study needs.
Keywords: Leishmania (利什曼原虫)Graphical overview
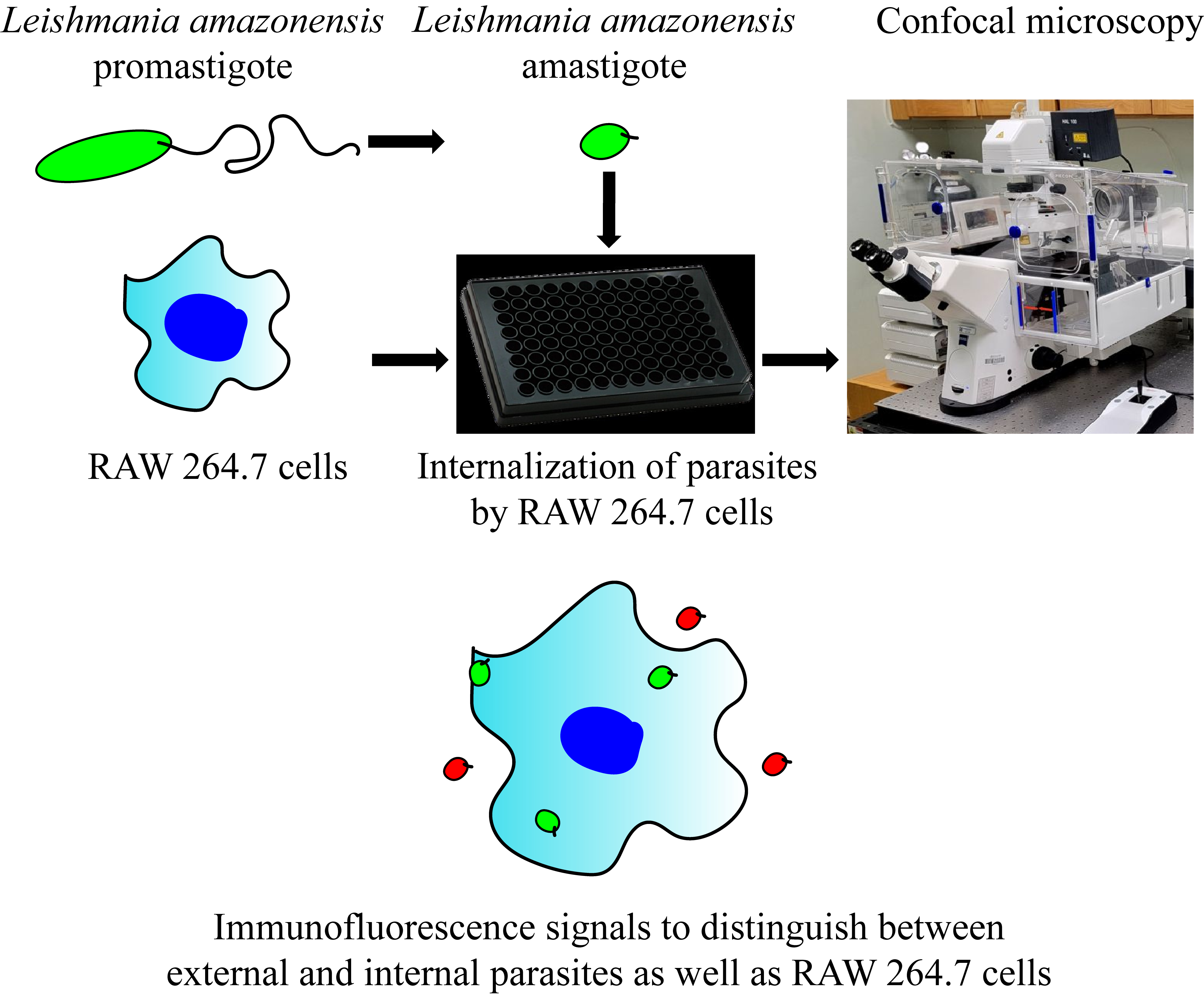
Background
Leishmaniasis wreaks havoc in a population of approximately 2 million annually across 90 endemic countries, being caused by < 20 different species of the parasite Leishmania [1]. Depending on the infective species and host immune system function, the resulting pathology can include cutaneous, mucocutaneous, or visceral disease. A need for new, effective drugs is demonstrated by the inadequacies of existing treatments since current drugs for leishmaniasis suffer serious limitations such as poor efficacy, toxicity, and resistance [2,3].
In their sand fly vector, Leishmania exist as free, motile promastigotes with an elongated cell shape as well as a long flagellum. After transmission into the mammalian host, they multiply, undergo transformation, and subsequently survive as intracellular amastigotes in the phagolysosomal compartment of macrophages (MΦ) and other phagocytes [4]. Amastigotes exhibit significantly lower cell surface-to-volume ratio, and the flagellum is reduced to a tiny distended tip [5]. Although both forms are capable of infecting mammalian cells, only amastigotes can spread and eventually sustain the infection in the host [6,7]. Conversely, for many (although not all) Leishmania species, only promastigotes can be cultured without mammalian cells (axenically). As such, although the process of Leishmania uptake by human MΦ has been studied using confocal microscopy, the relative intractability of amastigotes for most species has resulted in a predominant focus on promastigotes, which in turn overlooks many of the intricacies involved in Leishmania’s interactions with host MΦ [8]. Since the host cell’s response to promastigotes and amastigotes varies significantly, a more comprehensive understanding of the entire life cycle is required for effective pathogenesis studies and drug discovery strategies.
Here, we provide a multi-color immunofluorescence protocol for studying the uptake of Leishmania by MΦ with microscopy. This protocol has been adapted from a number of prior studies in the parasitology field. However, in combination with various culture techniques, our protocol, encompassing both promastigotes and amastigotes in the experimental setup, has further expanded approaches to studies characterizing host–pathogen interactions. Furthermore, our protocol allows many ways in which it can be adapted to the situation at hand, including characterization of higher-order subcellular structures during parasite internalization or high-throughput screening and analysis for drug discovery. Our expanded methodology provides a foundation for a better understanding of the pathogenesis of leishmaniasis and the development of more efficacious and targeted therapeutic interventions.
Materials and reagents
Biological materials
RAW 264.7 cells (ATCC, catalog number: TIB-71)
.Leishmania (L.) amazonensis promastigotes (strain IFLA/BR/67/PH8) (Norma W. Andrews, University of Maryland, College Park, MD)
L. amazonensis-mNeon, expressing bright monomeric green fluorescent protein, mNeonGreen [9]
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), with 4500 mg/L glucose, L-glutamine, sodium pyruvate, and sodium bicarbonate (Sigma-Aldrich, catalog number: D6429), store at 4 °C
Benchmark fetal bovine serum (FBS), heat inactivated (GeminiBio, catalog number: 100-106-500-HI), store at -20 °C
Penicillin/streptomycin solution (GeminiBio, catalog number: 400-109-100), store at -20 °C
Hygromycin B solution (50 mg/mL) (GeminiBio, catalog number: 400-123-020), store at 4 °C
Schneider’s Drosophila medium (Sigma-Aldrich, catalog number: S9895), store at 4 °C
Hemin (Sigma-Aldrich, catalog number: H9039), store at 4 °C
Sodium hydroxide solution, 10 M in water (Sigma-Aldrich, catalog number: 72068)
Phosphate buffered saline (PBS), pH 7.4, liquid, sterile-filtered, suitable for cell culture (Sigma-Aldrich, catalog number: 806552), store at 4 °C
Formaldehyde, 37% in water (Sigma-Aldrich, catalog number: F1635)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number A7906), store at 4 °C
Triton X-100, molecular biology grade (Sigma-Aldrich, catalog number: T8787)
Mouse anti-p8 antibody, generated from hybridoma cell line generously donated from McMahon-Pratt laboratory, Yale University; available upon request, store at -20 °C
Anti-mouse IgG (H+L), F(ab')2 fragment, CF-488A-conjugate antibody produced in goat (Sigma-Aldrich, catalog number: SAB4600388), store at -20 °C
Anti-mouse IgG (H+L), highly cross-adsorbed, CF-568-conjugate antibody produced in donkey (Sigma-Aldrich, catalog number: SAB4600075), store at -20 °C
Hoechst 33342 solution, 20 mM (Thermo Fisher, catalog number: 62249), store at 4 °C
Alexa FluorTM 647 Phalloidin (Thermo Fisher, catalog number: A22287), store at -20 °C
Solutions
Complete RAW 264.7 cell media (see Recipes)
50 mM sodium hydroxide (see Recipes)
Hemin solution (see Recipes)
Complete promastigote media (see Recipes)
Complete amastigote media (see Recipes)
Fixing solution (see Recipes)
Blocking solution (see Recipes)
Permeabilization solution (see Recipes)
Recipes
Complete RAW 264.7 cell media, final pH 7.4 (4 °C storage)
Reagent Final concentration Quantity DMEM n/a 440 mL FBS 10% 50 mL Penicillin/streptomycin solution 1× 5 mL Hygromycin 1× 5 mL Total n/a 500 mL 50 mM sodium hydroxide
Reagent Final concentration Quantity 10 M sodium hydroxide 50 mM 0.5 mL Water n/a 99.5 mL Total n/a 100 mL Hemin solution (4 °C storage)
Reagent Final concentration Quantity Hemin 2.5 mg/mL 25 mg 50 mM sodium hydroxide n/a 10 mL Total n/a 10 mL Complete promastigote media, final pH 7.4 (4 °C storage)
Reagent Final concentration Quantity Schneider’s Drosophila medium n/a 835 mL FBS 15% 150 mL Penicillin/streptomycin solution 10% 10 mL Hygromycin 1× 5 mL Total n/a 1000 mL Complete amastigote media, final pH 5.4 (4 °C storage)
Reagent Final concentration Quantity Schneider’s Drosophila medium n/a 785 mL FBS 20% 200 mL Penicillin/streptomycin solution 1× 10 mL Hemin solution (Recipe 3) 0.5% 5 mL Total n/a 1,000 mL Adjust final pH to 5.4.
Fixing solution (-20 °C storage)
Reagent Final concentration Quantity Formaldehyde, 37% in water 4% 5 mL 1× PBS n/a 45 mL Total n/a 50 mL Blocking solution (4 °C storage)
Reagent Final concentration Quantity BSA 5% 2.5 g 1× PBS n/a 50 mL Total n/a 50 mL Permeabilization solution
Reagent Final concentration Quantity Triton X-100 5% 0.5 mL Blocking solution n/a 9.5 mL Total n/a 10 mL
Laboratory supplies
Sterile 15 mL polypropylene centrifuge tubes (any vendor)
Sterile 50 mL polypropylene centrifuge tubes (any vendor)
Nalgene rapid-flow sterile disposable filter units 1 L (Thermo Scientific, catalog number: 5670020)
Sterile nuclease-free filter tips (10, 200, and 1,000 μL) (any vendor)
Vented flask surface area 25 cm2 (any vendor)
Sterile cell culture dish, 100 mm (Greiner Bio-One, catalog number: 664160)
Sterile cell scraper (Falcon, catalog number: 353089)
PhenoPlate 96-well, black, optically clear flat-bottom, tissue-culture treated microplate (PerkinElmer, catalog number: 6055300)
Disposable plastic serological pipettes, sterile, individually wrapped, 5 mL, 10 mL, 25 mL capacity (any vendor)
Hemocytometer (Hausser Scientific, catalog number: 3200)
Microcentrifuge 1.7 mL tubes (Corning, Axygen®, catalog number: MCT-175-C-S)
Bleach (Clorox)
50 mL reagent reservoir (Corning, catalog number: 4870)
PoseidonTM 12 channel pipettor P200, adjustable volume, 20–200 µL (Genesee Scientific, catalog number: 33-GSC-12P200)
Plastic container for waste collection, 1 L size (any vendor)
Equipment
Centrifuge 5810R (Eppendorf, catalog number: 022628187)
Inverted confocal microscope (Zeiss, model: LSM 800)
Software and datasets
Zen 2.3 SP1 FP3 (black) 64-bit (Carl Zeiss), licensed
GraphPad Prism Version 10.1.2 (324), licensed
ImageJ 1.53t (NIH, https://imagej.net/ij/download.html)
Microsoft Excel 2016 (16.0.5422.1000) MSO 32-bit, licensed
Adobe Illustrator 28.1 64-bit, licensed
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Datta, A., Barrie, U. and Wetzel, D. M. (2024). A Multi-Color Immunofluorescence Assay to Distinguish Intracellular From External Leishmania Parasites. Bio-protocol 14(11): e5009. DOI: 10.21769/BioProtoc.5009.
分类
微生物学 > 微生物-宿主相互作用 > 原生生物
细胞生物学 > 细胞成像 > 共聚焦显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link