- EN - English
- CN - 中文
Quantitative Measurement of Plasma Membrane Protein Internalisation and Recycling in Heterogenous Cellular Samples by Flow Cytometry
流式细胞术在异质细胞样本中定量测量质膜蛋白内吞和回收
发布: 2024年05月05日第14卷第9期 DOI: 10.21769/BioProtoc.4986 浏览次数: 2162
评审: Keisuke TabataYu Hui KangAndrea GramaticaAnonymous reviewer(s)

相关实验方案
外周血中细胞外囊泡的分离与分析方法:红细胞、内皮细胞及血小板来源的细胞外囊泡
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
2025年11月05日 1324 阅读
用于比较人冷冻保存 PBMC 与全血中 JAK/STAT 信号通路的双磷酸化 CyTOF 流程
Ilyssa E. Ramos [...] James M. Cherry
2025年11月20日 2122 阅读
Abstract
Plasma membrane proteins mediate important aspects of physiology, including nutrient acquisition, cell–cell interactions, and monitoring homeostasis. The trafficking of these proteins, involving internalisation from and/or recycling back to the cell surface, is often critical to their functions. These processes can vary among different proteins and cell types and states and are still being elucidated. Current strategies to measure surface protein internalisation and recycling are typically microscopy or biochemical assays; these are accurate but generally limited to analysing a homogenous cell population and are often low throughput. Here, we present flow cytometry–based methods involving probe-conjugated antibodies that enable quantification of internalisation or recycling rates at the single-cell level in complex samples. To measure internalisation, we detail an assay where the protein of interest is labelled with a specific antibody conjugated to a fluorescent oligonucleotide-labelled probe. To measure recycling, a specific antibody conjugated to a cleavable biotin group is employed. These probes permit the differentiation of molecules that have been internalised or recycled from those that have not. When combined with cell-specific marker panels, these methods allow the quantitative study of plasma membrane protein trafficking dynamics in a heterogenous cell mixture at the single-cell level.
Key features
• These assays allow sensitive quantification of internalised or recycled surface molecules using oligonucleotide or cleavable biotin-conjugated probes, respectively, and detected by flow cytometry.
• They can be adapted to any membrane protein that transits via the cell surface and for which a specific purified antibody is available.
• The dynamics of a cell surface protein can be measured in heterogenous cell populations simultaneously, including various cellular activation states.
• The internalisation assay builds upon the method developed by Liu et al. [1,2] and extends its application to heterogenous human peripheral blood mononuclear cells.
• These assays have been extensively used on suspension cells but have not been tested on adherent cells.
Keywords: Plasma membrane (质膜)Background
Cell surface proteins mediate many important physiological functions. How long they dwell at the cell surface is critical to their activity, and this is defined by a dynamic process of internalisation and often recycling from within the endosomal compartment back to the plasma membrane. For example, diverse cells display major histocompatibility complex (MHC) proteins, which allow patrolling T lymphocytes to monitor for pathogens or cancer [3]. MHC proteins are regulated by distinct mechanisms, and this can vary depending on the cell type or activation state [4–6]. Such protein trafficking is still being understood for diverse cell types; therefore, accurate methods are required.
Two major strategies to accurately quantify surface protein internalisation and recycling are microscopy-based or biochemical assays, but these have limitations. Microscopy allows the visualisation of subcellular localisation of the labelled surface molecule. However, it can be challenging to track molecules of low abundance or to differentiate internalised from surface molecules, especially at low resolution [7]. Further, microscopy can only accommodate a limited number of cells, and biochemical methods generally observe the sum of a single-cell population, making the analysis of specific cells in heterogenous samples impractical.
Here, we describe methods to quantitate the internalisation and recycling of membrane proteins in different cell types using functional probes conjugated to specific antibodies and detected by flow cytometry [4]. When combined with specific markers to differentiate cell types, protein dynamics can be analysed in a complex cell mixture at the single-cell level. Similar flow cytometry–based assays involve acid stripping the cell surface, but these have been shown to be harsh on cells, destroying some epitopes that prevent cell phenotyping, and lead to undesired cellular activation [8–11]. Here, we detail assays that allow gentle, rapid, and quantitative measurement of cell surface protein trafficking.
For internalisation, we detail the method developed by Liu et al. [1,2]. A fluorophore-labelled oligonucleotide is conjugated to a surface protein-specific antibody, termed the fluorescent internalisation probe (FIP) (Figure 1A). Cells are labelled and then allowed to internalise their labelled surface cargo. The fluorescence of the FIP-labelled proteins remaining on the cell surface is quenched with a complementary oligonucleotide-conjugated quencher, leaving only the internalised molecules fluorescent. Hence the cellular fluorescence remaining after quenching reflects the internalised molecules at each time point (Figure 1A).
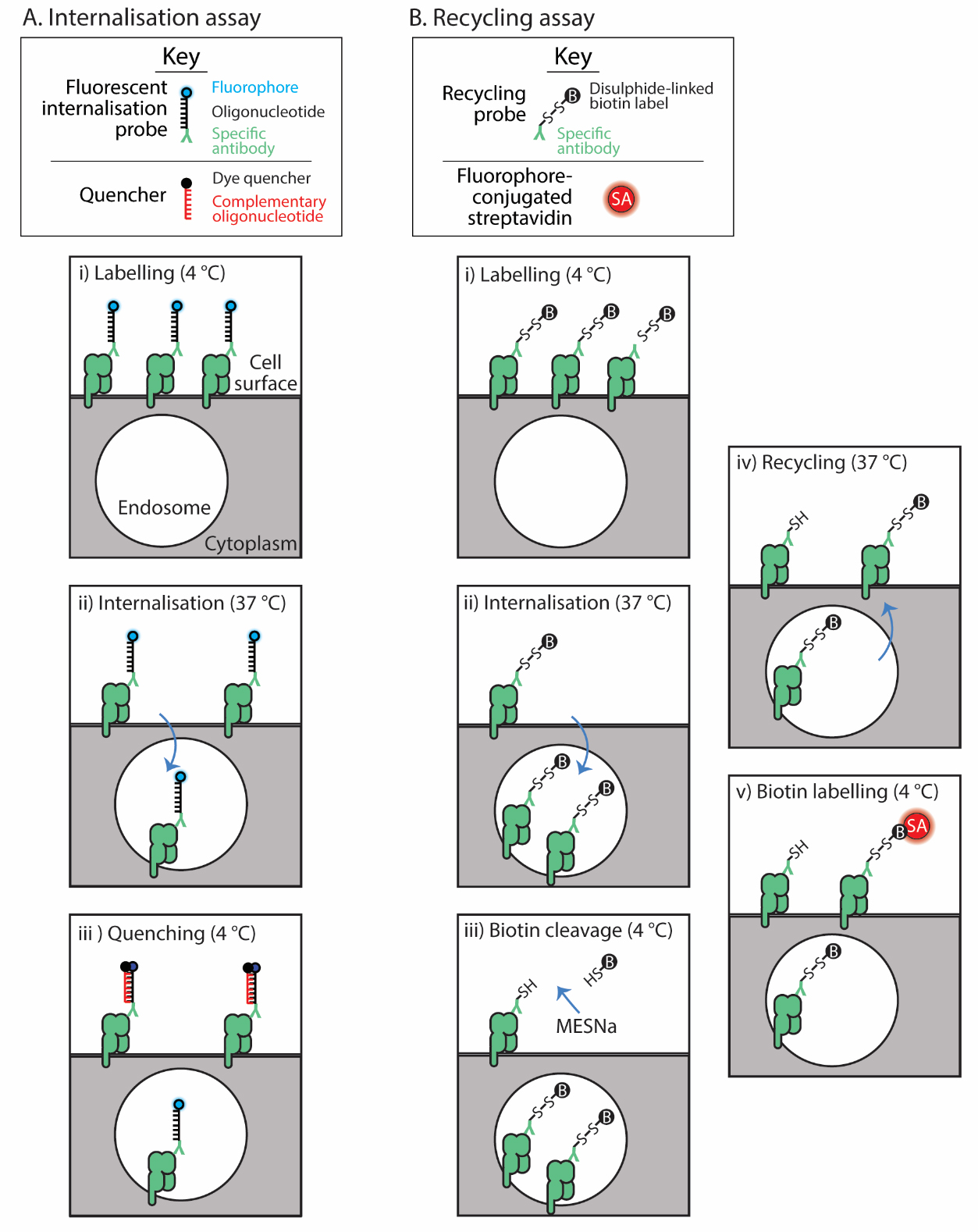
Figure 1. Assay schematics. The internalisation assay (A) employs the fluorescent internalisation probe to label the surface protein of interest (i); then, after a cohort of these internalise (ii), a quencher distinguishes those that remain on the surface from those internalised (iii). The recycling assay (B) uses a recycling probe to label the surface protein (i); then, these are allowed to internalise (ii). The label on the probe bound to those remaining on the surface is cleaved (iii) and then recycling is allowed to occur (iv). Finally, those molecules that recycled are detected with fluorescently labelled streptavidin (SA) (v).
For recycling, a biochemical assay is adapted for flow cytometry [12,13]. Surface proteins are labelled with an antibody conjugated to a cleavable biotin group (Figure 1B). Proteins are allowed to internalise; then, the biotin tag is cleaved from non-internalised proteins with a membrane-impermeable reducing reagent. Proteins are allowed to recycle, and then any biotin probe–labelled proteins that re-emerge from inside the cell are detected with fluorophore-conjugated streptavidin. This assay then calculates the proportion of original surface molecules that recycle after a defined period of internalisation [4,14].
With these methods, we have measured the internalisation and recycling of the MHC class I-related protein 1 (MR1) and transferrin receptor in a range of cell types including peripheral blood mononucleated cells (PBMC) [4,14]. These methods can be used to study any membrane protein of interest in suspension cells but may also be adapted to adherent cells.
Materials and reagents
Biological materials
Human peripheral mononucleated cells (PBMCs) are isolated from buffy coats using Ficoll Paque PLUS (Cytiva, catalog number: 17144002) according to the manufacturer’s instructions. Buffy coats from healthy human donors were obtained from the Australia Red Cross Blood Service with written and informed consent and with ethics approval from the University of Melbourne Human Research and Ethics Committee (#1035100).
Reagents
RPMI 1640 medium (Gibco, catalog number: 11875093)
Fetal bovine serum (FBS) (Gibco, catalog number: 5256701). Heat-inactivate at 56 °C for 30 min with interval mixing and store at -20 °C
Ethylenediaminetetraacetic acid-balanced salt solution (EDTA-BSS): 147 mM NaCl, 3.6 mM KCl, 0.01 mM KH2PO4, 0.02 mM K2HPO4, 14.5 mM HEPES, 6 mM EDTA (prepared in-house by dissolving each chemical in distilled water)
Phosphate buffered saline (PBS), pH 7.4 (Thermo Fisher, catalog number: 10010023)
10 mM sodium bicarbonate, pH 8.0, diluted from 1 M (Thermo Fisher Scientific, catalog number: J62495.AP)
Purified antibody specific for the cell surface protein to be assessed for internalisation or recycling (at a concentration of at least 2 mg/mL)
Cell type–specific surface markers for cell phenotyping, e.g.:
PerCP conjugated anti-CD3 (BD, catalog number: 345766)
BUV805 conjugated anti-CD14 (BD Horizon, catalog number: 612902)
Fixable viability dye eFluor780 (eBioscience, catalog number: 65-0865-14)
Click-iTTM sDIBO Alkynes (Invitrogen, catalog number: C20025), dissolved in anhydrous DMSO at 1 mg/mL
Cy5-oligo-azide (5' Cy5-TCA GTT CAG GAC CCT CGG CT-N3 3') (Integrated DNA Technologies), dissolved at 150 μM in nuclease-free water and stored at -20 °C
Quencher (5' AGC CGA GGG TCC TGA ACT GA-BHQ2 3') (Integrated DNA Technologies), dissolved at 600 μM in nuclease-free water and stored at -20 °C
EZ-LinkTM Sulfo-NHS-SS-Biotin (Thermo Fisher Scientific, catalog number: 21331)
Streptavidin-phycoerythrin (PE) (BioLegend, catalog number: 405203)
1 M Tris buffer, pH 8.0 (prepared in-house by dissolving Tris in distilled water and then adjusting pH with 1 M hydrochloric acid)
Sodium chloride (NaCl) (Spectrum Chemical, catalog number: SO160)
0.5 M EDTA, pH 8.0 (prepared in-house by stirring the required amount of EDTA powder in distilled water and then adjusting pH with solid sodium hydroxide pellets)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7906)
Sodium 2-mercaptoethanesulfonate (MESNa) (Sigma-Aldrich, catalog number: M1511)
Zeba spin desalting column (7K, MWCO 0.5 mL) (Thermo Fisher Scientific, catalog number: 89882)
Amicon Ultra centrifugal filters (30 kDa, MWCO 0.5 mL) (Millipore, catalog number: UFC5030)
Low-protein binding 1.5 mL centrifuge tubes (Thermo Scientific, catalog number: 90410)
Round bottom 96-well plates (Corning, catalog number: 3799)
Cell culture media (see Recipes)
Staining buffer (see Recipes)
Recycling assay buffer 1 (see Recipes)
Recycling assay buffer 2 (see Recipes)
MESNa incubation buffer (see Recipes)
Cell surface marker staining solution (see Recipes)
Recipes
Cell culture media (500 mL)
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume RPMI 1640 n/a 450 mL FBS 10% (v/v) 50 mL Total n/a 500 mL Staining buffer (510 mL)
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume EDTA-BSS n/a 500 mL FBS 2% (v/v) 10 mL Total n/a 510 mL Recycling assay buffer 1 (500 mL)
Note: Dissolve the NaCl completely in milli-Q water before adding to the solution. Store at room temperature.
Reagent Final concentration Quantity or Volume 1 M Tris buffer, pH 8.0 50 mM 25 mL NaCl 100 mM 2.9 g 0.5 M EDTA, pH 8.0 1 mM 1 mL Milli-Q water Top up to 500 mL Total n/a 500 mL Recycling assay buffer 2 (50 mL)
Note: Prepare on the day of the assay. Scale down or up as required.
Reagent Final concentration Quantity or Volume Recycling assay buffer 1 n/a 50 mL BSA 0.2 % (w/v) 0.1 g Total n/a 50 mL MESNa incubation buffer (2 mL)
Note: Prepare on the day of the assay. Scale down or up as required.
Reagent Final concentration Quantity or Volume Recycling assay buffer 2 n/a 2 mL MESN 100 mM 0.033 g Total n/a 2 mL Cell surface marker staining solution
Note: Prepare on the day of the assay. Scale down or up as required. Replace with antibodies to differentiate your cells of interest.
Reagent Dilution Volume PerCP conjugated anti-CD3 1:50 20 μL BUV805 conjugated anti-CD14 1:200 5 μL Fixable viability dye 1:1,000 1 μL Staining buffer n/a 1,000 μL
Equipment
Air-cooled benchtop centrifuge (Beckman Coulter, model: Microfuge 22R Centrifuge)
Cell culture incubator at 37 °C and 5%–10% CO2 (dependent on the recommended CO2 level for your cells or media)
Flow cytometer (BD, model: LSR Fortessa FACS Calibur)
Software and datasets
FlowJo (version 10.4, 09/15/2017); can be purchased and downloaded online from https://www.flowjo.com/solutions/flowjo/downloads
Graph Pad Prism (version 10.1.0, 10/19/2023); can be purchased and downloaded online from https://www.graphpad.com
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Lim, H. J. and McWilliam, H. E. G. (2024). Quantitative Measurement of Plasma Membrane Protein Internalisation and Recycling in Heterogenous Cellular Samples by Flow Cytometry. Bio-protocol 14(9): e4986. DOI: 10.21769/BioProtoc.4986.
- Lim, H. J., Wubben, J. M., Garcia, C. P., Cruz-Gomez, S., Deng, J., Mak, J. Y. W., Hachani, A., Anderson, R. J., Painter, G. F., Goyette, J., et al. (2022). A specialized tyrosine-based endocytosis signal in MR1 controls antigen presentation to MAIT cells. J. Cell Biol. 221(12). https://doi.org/10.1083/jcb.202110125.
分类
细胞生物学 > 基于细胞的分析方法 > 内吞作用
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
细胞生物学 > 细胞信号传导 > 胞内信号传导
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









